Barium Chloride Reacts With Sodium Sulphate
News Leon
Mar 21, 2025 · 6 min read
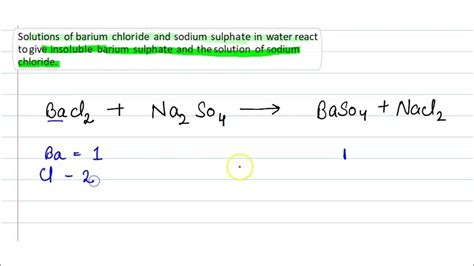
Table of Contents
Barium Chloride Reacts with Sodium Sulphate: A Deep Dive into a Classic Double Displacement Reaction
The reaction between barium chloride (BaCl₂) and sodium sulphate (Na₂SO₄) is a quintessential example of a double displacement reaction, also known as a metathesis reaction. Understanding this reaction provides a foundational understanding of chemical reactions, stoichiometry, and the properties of ionic compounds. This comprehensive article will explore the reaction in detail, covering its mechanism, applications, safety precautions, and its significance in chemistry education.
The Reaction: A Detailed Look
The reaction between barium chloride and sodium sulphate can be represented by the following balanced chemical equation:
BaCl₂(aq) + Na₂SO₄(aq) → BaSO₄(s) + 2NaCl(aq)
This equation shows that aqueous solutions of barium chloride and sodium sulphate react to produce solid barium sulphate (BaSO₄) and aqueous sodium chloride (NaCl). The (aq) denotes an aqueous solution (dissolved in water), and (s) signifies a solid precipitate.
The Mechanism: Ionic Interactions at Play
This reaction occurs because of the strong electrostatic attraction between the ions in the reactants. In aqueous solution, barium chloride and sodium sulphate dissociate into their constituent ions:
- BaCl₂(aq) → Ba²⁺(aq) + 2Cl⁻(aq)
- Na₂SO₄(aq) → 2Na⁺(aq) + SO₄²⁻(aq)
These ions are free to move around in the solution. When the two solutions are mixed, the barium ions (Ba²⁺) and sulphate ions (SO₄²⁻) encounter each other. The strong electrostatic attraction between the doubly charged barium cation and the doubly charged sulphate anion overcomes the attractive forces of hydration (the interaction between the ions and water molecules). This leads to the formation of an insoluble ionic compound, barium sulphate, which precipitates out of the solution as a white solid. The sodium (Na⁺) and chloride (Cl⁻) ions remain in solution as they form a soluble ionic compound, sodium chloride.
Driving Force: Formation of an Insoluble Precipitate
The driving force behind this reaction is the formation of the insoluble barium sulphate precipitate. This precipitation removes barium and sulphate ions from the solution, shifting the equilibrium towards the product side. The principle governing this is Le Chatelier's principle, which states that a system at equilibrium will shift to counteract any stress applied to it. The removal of ions through precipitation acts as a stress, pushing the reaction forward.
Properties of the Reactants and Products
Understanding the properties of the reactants and products is crucial for comprehending the reaction.
Reactants:
- Barium Chloride (BaCl₂): A white, crystalline solid that is highly soluble in water. It's a common laboratory reagent used in various chemical processes.
- Sodium Sulphate (Na₂SO₄): A white, crystalline solid also highly soluble in water. It finds use in various industries, including the paper and textile industries.
Products:
- Barium Sulphate (BaSO₄): A white, crystalline solid that is virtually insoluble in water. This low solubility is key to its applications, particularly in medical imaging.
- Sodium Chloride (NaCl): Common table salt, a white crystalline solid highly soluble in water.
Applications of the Reaction and its Products
The reaction between barium chloride and sodium sulphate, and the properties of its products, have various applications:
- Qualitative Analysis: This reaction is frequently used in qualitative inorganic analysis to identify the presence of either barium ions or sulphate ions in a solution. The formation of the white precipitate, barium sulphate, confirms the presence of both ions.
- Preparation of Barium Sulphate: While barium sulphate can be prepared through other methods, this reaction provides a straightforward method for its synthesis in a laboratory setting.
- Medical Imaging (Barium Sulfate): The low solubility and high X-ray opacity of barium sulphate make it ideal as a contrast agent in medical imaging, specifically in gastrointestinal tract examinations (barium swallow, barium enema). The insoluble nature of barium sulphate ensures that it does not get absorbed into the body.
- Industrial Applications (Sodium Sulphate & Sodium Chloride): Sodium sulphate and sodium chloride are widely used in various industries. Sodium sulphate finds applications in the paper, textile, and detergent industries, while sodium chloride is essential in numerous industrial processes and as a food preservative.
Safety Precautions
When performing this reaction or handling the chemicals involved, several safety precautions must be observed:
- Eye Protection: Always wear safety goggles to protect your eyes from splashes.
- Gloves: Wear appropriate chemical-resistant gloves to prevent skin contact with the chemicals.
- Lab Coat: A lab coat should be worn to protect your clothing.
- Proper Waste Disposal: Dispose of the waste according to the guidelines set by your institution or local regulations. Barium compounds, while generally considered low toxicity, should be handled with care.
Stoichiometry and Calculations
The balanced chemical equation allows us to perform stoichiometric calculations. For instance, if we know the amount of barium chloride reacted, we can calculate the theoretical yield of barium sulphate produced.
Example:
Let's say we react 10 grams of barium chloride with an excess of sodium sulphate. To find the theoretical yield of barium sulphate:
-
Convert grams of BaCl₂ to moles: The molar mass of BaCl₂ is approximately 208.23 g/mol. Therefore, 10 g BaCl₂ / 208.23 g/mol ≈ 0.048 moles BaCl₂.
-
Use the mole ratio: From the balanced equation, 1 mole of BaCl₂ produces 1 mole of BaSO₄. Thus, 0.048 moles BaCl₂ will produce 0.048 moles BaSO₄.
-
Convert moles of BaSO₄ to grams: The molar mass of BaSO₄ is approximately 233.38 g/mol. Therefore, 0.048 moles BaSO₄ * 233.38 g/mol ≈ 11.2 g BaSO₄.
This calculation shows that, theoretically, approximately 11.2 grams of barium sulphate should be produced from 10 grams of barium chloride, given an excess of sodium sulphate. In practice, the actual yield might be slightly lower due to various factors, such as incomplete reaction or loss of product during filtration.
Beyond the Basics: Exploring Related Concepts
This reaction provides a springboard for exploring other important concepts in chemistry:
- Solubility Rules: Understanding solubility rules helps predict whether a double displacement reaction will produce a precipitate.
- Net Ionic Equations: Writing a net ionic equation focuses on the ions directly involved in the reaction, omitting spectator ions (ions that do not participate in the reaction, in this case Na⁺ and Cl⁻). The net ionic equation for this reaction is: Ba²⁺(aq) + SO₄²⁻(aq) → BaSO₄(s)
- Equilibrium: The reaction reaches equilibrium when the rate of the forward reaction (formation of BaSO₄) equals the rate of the reverse reaction (dissociation of BaSO₄). However, due to the very low solubility of BaSO₄, the equilibrium lies heavily on the product side.
- Ksp (Solubility Product Constant): The Ksp value quantifies the solubility of barium sulphate. Its low Ksp value reflects the very low solubility of this compound.
Conclusion
The reaction between barium chloride and sodium sulphate is a seemingly simple yet profoundly instructive chemical reaction. It beautifully demonstrates fundamental concepts such as double displacement reactions, precipitation reactions, stoichiometry, and the importance of solubility rules. Its applications in qualitative analysis, medical imaging, and industrial processes highlight the significance of this seemingly basic chemical reaction in various fields. By understanding this reaction, we gain a deeper appreciation of the principles governing the behaviour of ionic compounds and the elegance of chemical reactions in the macroscopic world. Further exploration of related concepts will undoubtedly enhance your understanding of chemistry and related scientific disciplines.
Latest Posts
Latest Posts
-
Difference Between Molar Mass And Molecular Mass
Mar 27, 2025
-
Blood Pressure Is Highest In The And Lowest In The
Mar 27, 2025
-
What Are The Two Main Categories Of Computer Software
Mar 27, 2025
-
Which Statement Best Describes A Mixed Market Economy
Mar 27, 2025
-
What Is The Oxidizing Agent In The Following Reaction
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Barium Chloride Reacts With Sodium Sulphate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
