Are Anions Bigger Than Neutral Atoms
News Leon
Mar 20, 2025 · 5 min read
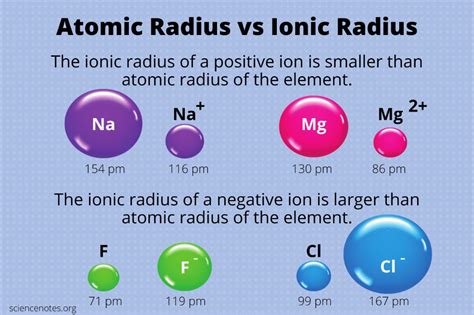
Table of Contents
Are Anions Bigger Than Neutral Atoms? A Deep Dive into Ionic Radii
Understanding the size of atoms and ions is fundamental to chemistry. This article delves into the fascinating world of ionic radii, specifically exploring the question: are anions bigger than neutral atoms? The short answer is a resounding yes, but the reasons behind this are complex and require a detailed examination of electron configuration and electrostatic forces.
Understanding Atomic and Ionic Radii
Before we delve into the comparison, let's define our terms. Atomic radius refers to the distance from the nucleus to the outermost electron shell of a neutral atom. This is not a fixed value, as electron clouds are probabilistic rather than precisely defined boundaries. Measurements usually represent an average distance.
Ionic radius, on the other hand, refers to the radius of an ion – an atom that has gained or lost electrons, resulting in a net electrical charge. Cations are positively charged ions (lost electrons), while anions are negatively charged ions (gained electrons).
The Impact of Electron Gain on Anionic Size
The key to understanding why anions are larger than their neutral atom counterparts lies in the addition of electrons. When a neutral atom gains an electron to form an anion, several factors contribute to the increase in size:
1. Increased Electron-Electron Repulsion:
The most significant factor is the increase in electron-electron repulsion. Adding an electron introduces an extra negative charge to the electron cloud. These negatively charged particles repel each other, causing the electron cloud to expand. The increased repulsion overcomes the attractive force of the nucleus, leading to a larger ionic radius.
This repulsion is not evenly distributed. The newly added electron enters the outermost electron shell, experiencing the least effective nuclear charge (the net positive charge experienced by an electron). The outer shell electrons are therefore less tightly held, resulting in a significant expansion.
2. Reduced Effective Nuclear Charge:
The effective nuclear charge (Z<sub>eff</sub>) is the net positive charge experienced by an electron after accounting for the shielding effect of other electrons. The addition of an electron increases the number of electrons, thereby increasing the shielding effect on the outer electrons. This reduces the effective nuclear charge experienced by each electron, weakening the attraction between the nucleus and the outer electrons. This weaker attraction further contributes to the expansion of the anion.
3. Increased Electron Shell:
In some cases, the addition of an electron may result in a complete electron shell. For instance, when a chlorine atom (Cl) gains an electron to become a chloride ion (Cl⁻), it fills its 3p subshell. This creates a more stable, fully occupied electron shell, resulting in a larger ionic radius compared to the neutral chlorine atom. This effect is amplified by the increased electron-electron repulsion and reduced effective nuclear charge.
Comparing Anions and Cations: A Size Contrast
It's important to contrast the size changes in anions with those in cations. While anions grow larger, cations become smaller than their neutral atoms. This is because removing an electron reduces electron-electron repulsion and increases the effective nuclear charge experienced by the remaining electrons. The stronger attraction pulls the remaining electrons closer to the nucleus, resulting in a smaller ionic radius.
Examples Illustrating Anionic Size Increase
Let's examine some specific examples to solidify our understanding:
-
Oxygen (O) and Oxide (O²⁻): A neutral oxygen atom has a significantly smaller radius than the oxide ion (O²⁻). Gaining two electrons substantially increases electron-electron repulsion and reduces the effective nuclear charge, leading to a considerably larger ionic radius.
-
Chlorine (Cl) and Chloride (Cl⁻): Similar to oxygen, the chloride ion (Cl⁻) is notably larger than the neutral chlorine atom. The addition of a single electron increases repulsion and decreases effective nuclear charge, causing the expansion.
-
Sulfur (S) and Sulfide (S²⁻): The sulfide ion (S²⁻) demonstrates an even greater expansion compared to its neutral atom counterpart due to the addition of two electrons. The effect is amplified compared to the chloride ion due to sulfur's larger number of electrons and protons.
Factors Influencing Anionic Size Variations
While the general principle holds true – anions are larger than their neutral atoms – the degree of size increase can vary depending on several factors:
-
Nuclear Charge: A higher nuclear charge will exert a stronger attractive force on electrons, mitigating the expansion caused by the added electron(s).
-
Number of Electrons Added: Gaining more electrons leads to greater electron-electron repulsion and a more substantial increase in size.
-
Electron Shell: The addition of electrons leading to a complete electron shell contributes significantly to the size increase.
Practical Applications of Understanding Anionic Size
The understanding of ionic radii has wide-ranging applications in various fields of chemistry and materials science:
-
Crystal Structure Prediction: The relative sizes of anions and cations are crucial in predicting the structure of ionic crystals. The arrangement of ions in a lattice depends on their sizes and charges, influencing the properties of the material.
-
Solubility and Reactivity: Ionic size influences the solubility and reactivity of ionic compounds. Larger anions may be less tightly bound to cations, resulting in higher solubility.
-
Catalysis: The size of anions can play a critical role in catalytic processes, where the interaction between ions and reactants is important.
-
Biochemistry: The size and charge of anions influence their interactions with biomolecules, impacting biological processes.
Conclusion: A Definitive Yes
In conclusion, the answer to the question "Are anions bigger than neutral atoms?" is unequivocally yes. The addition of electrons to a neutral atom to form an anion increases electron-electron repulsion, reduces the effective nuclear charge, and in many cases leads to the completion of a stable electron shell. These factors combine to cause a significant expansion of the electron cloud, resulting in a larger ionic radius compared to the neutral atom. Understanding this fundamental principle is essential for a comprehensive grasp of atomic structure, chemical bonding, and numerous applications in various scientific fields. Further research into specific elements and their ionic states provides a deeper understanding of these complexities.
Latest Posts
Latest Posts
-
What Is Not A Form Of Energy
Mar 21, 2025
-
120 F Is What In Celsius
Mar 21, 2025
-
Why Is Blood Considered To Be A Connective Tissue
Mar 21, 2025
-
Which Molecule Contains Sp Hybridized Orbitals
Mar 21, 2025
-
I Have Taken Notes On Your Book However
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Are Anions Bigger Than Neutral Atoms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
