An Endothermic Reaction Is One That
News Leon
Mar 23, 2025 · 6 min read
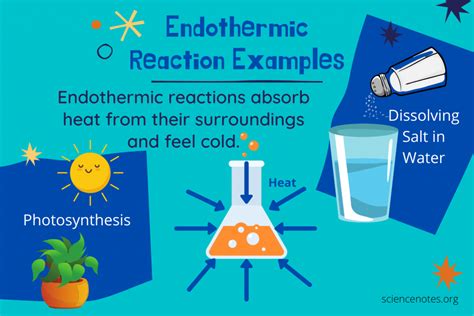
Table of Contents
An Endothermic Reaction Is One That… Absorbs Energy from its Surroundings
Endothermic reactions are a fundamental concept in chemistry, playing a crucial role in various natural processes and industrial applications. Understanding what defines an endothermic reaction, its characteristics, examples, and significance is key to grasping the broader principles of thermodynamics and chemical reactions. This comprehensive guide delves deep into the subject, providing a thorough understanding suitable for students, enthusiasts, and professionals alike.
Defining Endothermic Reactions: The Energy Absorption Process
An endothermic reaction is one that absorbs heat energy from its surroundings. This absorption of energy causes a decrease in the temperature of the surroundings. Think of it like this: the reaction is "sucking up" energy from its environment to proceed. This contrasts sharply with exothermic reactions, which release heat into their surroundings. The energy absorbed is used to break the chemical bonds in the reactants, allowing the formation of new bonds in the products. This process always results in a net increase in the system's enthalpy (ΔH > 0), meaning the products have a higher energy content than the reactants.
The Role of Enthalpy and Activation Energy
Enthalpy (H) is a thermodynamic property representing the total heat content of a system at constant pressure. In endothermic reactions, the enthalpy of the products is greater than the enthalpy of the reactants. This difference is the enthalpy change (ΔH), and it is positive for endothermic processes.
Activation energy (Ea) is the minimum energy required to initiate a chemical reaction. Even though endothermic reactions absorb energy overall, they still require activation energy to get started. This energy is needed to break the initial bonds in the reactants. Once the reaction is initiated, it continues to absorb energy from the surroundings.
Identifying Endothermic Reactions: Key Characteristics and Indicators
Several key characteristics help identify endothermic reactions. Recognizing these indicators is essential in both laboratory settings and real-world applications.
1. Temperature Decrease: The Most Obvious Sign
The most prominent sign of an endothermic reaction is a decrease in the temperature of the surroundings. This is because the reaction absorbs heat from its environment, causing a noticeable cooling effect. You might feel the reaction vessel become colder to the touch.
2. Positive Enthalpy Change (ΔH): The Thermodynamic Marker
A positive enthalpy change (ΔH > 0) is the definitive thermodynamic indicator of an endothermic reaction. This signifies that the reaction has absorbed heat from its surroundings. This is often calculated or experimentally measured using calorimetry.
3. Endothermic Processes Often Require External Heat Input
Many endothermic reactions require a continuous supply of energy to proceed. Without this external energy source, the reaction will not proceed or will proceed very slowly. This is because the reaction needs a constant influx of energy to overcome the activation energy and keep absorbing energy from its surroundings.
4. Visual Indicators: Some Reactions Exhibit Visible Changes
While not all endothermic reactions exhibit visual changes, some may show signs like dissolution of a solid in a liquid, melting of ice, or sublimation of a solid. These processes involve overcoming intermolecular forces, requiring energy input.
Real-World Examples of Endothermic Reactions: Diverse Applications
Endothermic reactions are prevalent across diverse fields, from everyday phenomena to complex industrial processes. Here are some notable examples:
1. Photosynthesis: The Foundation of Life
Photosynthesis, the process by which plants convert light energy into chemical energy in the form of glucose, is a classic example of an endothermic reaction. Plants absorb light energy from the sun, which drives the reaction to convert carbon dioxide and water into glucose and oxygen.
Equation: 6CO₂ + 6H₂O + Light Energy → C₆H₁₂O₆ + 6O₂
2. Melting Ice: A Common Endothermic Change
The melting of ice is another easily observable endothermic process. Energy from the surroundings is absorbed to break the hydrogen bonds holding the water molecules in a rigid crystalline structure, transforming ice into liquid water.
Equation: H₂O(s) + Heat → H₂O(l)
3. Dissolving Ammonium Nitrate in Water: A Cooling Effect
Dissolving ammonium nitrate (NH₄NO₃) in water is a common endothermic reaction frequently used in cold packs. The dissolution process absorbs heat from the surroundings, resulting in a significant temperature decrease.
Equation: NH₄NO₃(s) + H₂O(l) → NH₄⁺(aq) + NO₃⁻(aq) + Heat
4. Electrolysis of Water: Splitting Water Molecules
Electrolysis of water, the process of splitting water molecules into hydrogen and oxygen gases using electricity, is an endothermic reaction. Electrical energy is needed to break the strong covalent bonds in the water molecule.
Equation: 2H₂O(l) + Electrical Energy → 2H₂(g) + O₂(g)
5. Cooking an Egg: Denaturation of Proteins
While not purely endothermic, cooking an egg involves multiple processes, including the denaturation of proteins. This denaturation requires energy input, making it partially endothermic.
6. Baking a Cake: Chemical Changes Requiring Heat
Similar to cooking an egg, baking a cake involves numerous chemical and physical changes, many of which require heat input, making aspects of the baking process endothermic.
Endothermic Reactions in Industrial Processes: Applications and Implications
Endothermic reactions play a significant role in various industrial processes. Understanding their characteristics is critical for efficient process design and control.
1. Production of Ammonia: The Haber-Bosch Process
The Haber-Bosch process for ammonia synthesis is a complex reaction involving both endothermic and exothermic steps. While the overall reaction is exothermic, certain steps within the process are endothermic.
2. Chemical Separations and Purifications: Utilizing Endothermic Processes
Many separation and purification techniques in the chemical industry rely on endothermic processes. For instance, the separation of different components in a mixture might involve endothermic processes.
3. Material Synthesis: Utilizing Endothermic Reactions
Several industrial materials are synthesized using reactions that involve endothermic steps. Understanding these steps is essential in optimizing the synthesis and properties of the final materials.
Understanding and Controlling Endothermic Reactions: Practical Considerations
The efficient use of endothermic reactions in various settings requires a good understanding of their behavior and how to manage them.
1. Heat Management: Providing Sufficient Energy Input
In many cases, providing sufficient energy input to drive the endothermic reaction is crucial. This may involve using heat sources, light sources, or electrical energy.
2. Reaction Rate Optimization: Balancing Energy Input and Reaction Kinetics
Finding the optimal balance between energy input and reaction rate is important for efficient and safe operation. Factors such as temperature, pressure, and catalysts can significantly influence the reaction rate.
3. Safety Considerations: Handling Temperature Changes and Potential Hazards
Since endothermic reactions often involve temperature changes, appropriate safety precautions should be taken to handle potential hazards. This may involve using protective equipment, proper ventilation, and emergency procedures.
Conclusion: The Significance of Endothermic Reactions in Chemistry and Beyond
Endothermic reactions are integral to numerous natural processes and industrial applications. From the fundamental process of photosynthesis sustaining life on Earth to industrial applications in material synthesis and chemical separations, understanding these reactions is crucial. Their defining characteristic – the absorption of energy from their surroundings – distinguishes them from exothermic reactions and highlights their unique role in the broader landscape of chemical transformations. By understanding their characteristics, controlling them effectively, and appreciating their significance, we can unlock their immense potential in various fields. Further research into the intricacies of endothermic reactions promises continued advancements in technology and a deeper understanding of the natural world.
Latest Posts
Latest Posts
-
Like Charges Repel And Unlike Charges Attract
Mar 25, 2025
-
What Are Three Properties Of A Magnet
Mar 25, 2025
-
Which Of The Following Is An Example Of Polysaccharide
Mar 25, 2025
-
Lysosomes Are Membrane Bound Vesicles That Arise From The
Mar 25, 2025
-
Calculate The Radius Of Gyration Of A Cylindrical Rod
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about An Endothermic Reaction Is One That . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
