An Atom That Has Lost An Electron Becomes
News Leon
Mar 31, 2025 · 7 min read
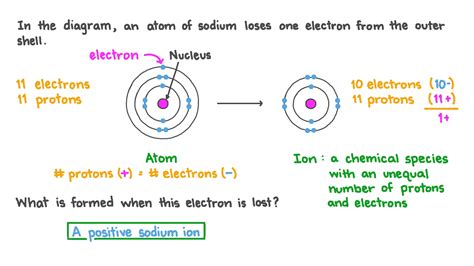
Table of Contents
An Atom That Has Lost an Electron Becomes… an Ion! Understanding Ionization and its Effects
When an atom loses an electron, it undergoes a fundamental transformation, becoming a positively charged ion, also known as a cation. This process, called ionization, plays a crucial role in numerous chemical and physical phenomena, shaping the behavior of matter at both microscopic and macroscopic levels. Understanding ionization is key to grasping concepts ranging from the formation of ionic bonds to the operation of advanced technologies like plasma displays. This article will delve into the details of ionization, exploring its causes, consequences, and significant applications across various scientific disciplines.
What Happens When an Atom Loses an Electron?
Atoms, the fundamental building blocks of matter, consist of a nucleus containing protons (positively charged) and neutrons (neutral), surrounded by a cloud of orbiting electrons (negatively charged). The number of protons determines the atom's identity (its atomic number) and defines the element. In a neutral atom, the number of protons equals the number of electrons, resulting in a net charge of zero.
However, this balance can be disrupted. When an atom loses one or more electrons, the number of protons exceeds the number of electrons, leading to a net positive charge. This positively charged atom is now called a cation. The magnitude of the positive charge depends on the number of electrons lost; losing one electron creates a +1 ion, losing two creates a +2 ion, and so on.
The process of electron removal is ionization, and the resulting ion is said to be ionized. This isn't a simple process; it requires a significant amount of energy to overcome the electrostatic attraction between the positively charged nucleus and the negatively charged electron.
Causes of Ionization
Several mechanisms can trigger ionization:
1. High Temperatures:
At extremely high temperatures, atoms gain sufficient kinetic energy for their electrons to overcome the attractive force of the nucleus and escape. This is common in stars and plasmas. The intense heat provides the energy needed for ionization, leading to a mixture of ions and free electrons. This thermal ionization is responsible for the characteristic properties of plasmas – a highly energized state of matter often called the fourth state of matter.
2. Collision with Other Particles:
Atoms can be ionized through collisions with other energetic particles, such as electrons, protons, or other ions. If the colliding particle possesses enough kinetic energy, it can transfer energy to an electron in the target atom, exceeding its binding energy and causing its ejection. This is a frequent mechanism in various environments, from particle accelerators to the Earth's upper atmosphere where cosmic rays cause ionization.
3. Electromagnetic Radiation:
Exposure to high-energy electromagnetic radiation, such as X-rays or gamma rays, can also ionize atoms. The photons in this radiation carry sufficient energy to knock electrons out of their atomic orbitals. This photoionization is crucial in various applications, including medical imaging and radiation therapy. Even ultraviolet (UV) light, while lower in energy than X-rays, can ionize certain atoms, particularly those with loosely bound electrons. This is a key process in the formation of the ozone layer, where UV radiation from the sun ionizes oxygen molecules.
4. Chemical Reactions:
Some chemical reactions can also lead to ionization. For instance, the formation of ionic compounds involves the transfer of electrons from one atom to another. The atom losing electrons becomes a cation, while the atom gaining electrons becomes an anion (a negatively charged ion). This process is driven by the electronegativity difference between the reacting atoms, with highly electronegative atoms readily accepting electrons.
Consequences of Ionization
The ionization of an atom has profound consequences, impacting its chemical and physical properties:
-
Change in Chemical Reactivity: Ions are highly reactive. The loss of an electron leaves the cation with an incomplete electron shell, making it prone to interact with other atoms or molecules to achieve a stable electron configuration. This drives many chemical reactions, especially in the formation of ionic compounds.
-
Change in Physical Properties: Ionization alters the physical properties of a substance. For example, ionized gases (plasmas) exhibit unique electrical conductivity and emissive properties, allowing for the creation of plasma displays and other technologies. The changes in electron configuration also influence the material's optical properties, potentially altering its color and light absorption/emission characteristics.
-
Formation of Ionic Compounds: The transfer of electrons between atoms during ionization results in the formation of ionic compounds. These compounds are held together by electrostatic attraction between oppositely charged ions, forming strong, stable structures with distinct properties. Examples include sodium chloride (NaCl, table salt) and magnesium oxide (MgO).
-
Creation of Free Radicals: In some cases, ionization can lead to the formation of free radicals – atoms or molecules with unpaired electrons. These highly reactive species can initiate chain reactions, damage biological molecules (like DNA), and contribute to various chemical processes.
Applications of Ionization
The principles of ionization find wide-ranging applications across various fields:
1. Mass Spectrometry:
Mass spectrometry utilizes ionization to identify and quantify molecules in a sample. By ionizing the molecules, they gain a charge and can be manipulated by electric and magnetic fields, separating them based on their mass-to-charge ratio. This is crucial in various analytical techniques employed in chemistry, biochemistry, and environmental science.
2. Plasma Displays:
Plasma displays leverage the emissive properties of ionized gases (plasmas). By exciting the gas molecules to a plasma state, they emit light, enabling the creation of vibrant and high-resolution displays. Although largely superseded by LCD and OLED technologies, plasma displays were groundbreaking for their high contrast and color accuracy.
3. Radiation Detectors:
Ionization detectors exploit the ability of ionizing radiation to create ion pairs in a gas. The resulting ions are collected, generating an electrical signal proportional to the radiation's intensity. These detectors are used in various applications, ranging from monitoring radiation levels in nuclear power plants to medical imaging.
4. Flame Ionization Detectors (FIDs):
FIDs are highly sensitive detectors used in gas chromatography (GC). When organic compounds are burned in a hydrogen flame, they ionize, creating a measurable current. This technique allows for the detection and quantification of trace amounts of organic compounds, crucial in various environmental and analytical applications.
5. Ion Thrusters:
Ion thrusters, used in spacecraft propulsion, accelerate ions to generate thrust. By ionizing a propellant (such as xenon), it is accelerated using electric fields, creating a low-thrust but highly efficient propulsion system, ideal for long-duration space missions.
Ionization Energy: A Measure of Electron Binding
The energy required to remove an electron from an atom is known as the ionization energy (IE). This is a crucial property reflecting the strength of the electrostatic attraction between the nucleus and the electron. The first ionization energy refers to the energy needed to remove the first electron, the second ionization energy to remove the second, and so on. Each subsequent ionization energy is generally higher than the previous one, as the remaining electrons are held more tightly by the increasingly positive ion.
Ionization energy varies across the periodic table, influenced by factors such as nuclear charge, atomic radius, and electron shielding. Elements with high ionization energies are less likely to lose electrons, while those with low ionization energies tend to readily ionize.
Conclusion
The ionization of an atom, resulting in the formation of a cation, is a fundamental process with far-reaching consequences across various scientific disciplines. Understanding the causes and effects of ionization is crucial for comprehending the behavior of matter in diverse environments, from the vastness of space to the intricacies of chemical reactions. The applications of ionization principles continue to expand, driving innovation in diverse fields, highlighting its importance in both fundamental science and advanced technology. The further exploration of ionization and its associated phenomena will undoubtedly lead to even more groundbreaking advancements in the future.
Latest Posts
Latest Posts
-
For Which Value Of X Is Abcd A Kite
Apr 01, 2025
-
64 To The Power Of 1 2
Apr 01, 2025
-
On The Galapagos Islands Charles Darwin Observed
Apr 01, 2025
-
A Population Is Composed Of Individuals Of
Apr 01, 2025
-
Suppose That An Electric Charge Is Produced
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about An Atom That Has Lost An Electron Becomes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
