Al Oh 3 H2so4 Balanced Equation
News Leon
Mar 21, 2025 · 6 min read
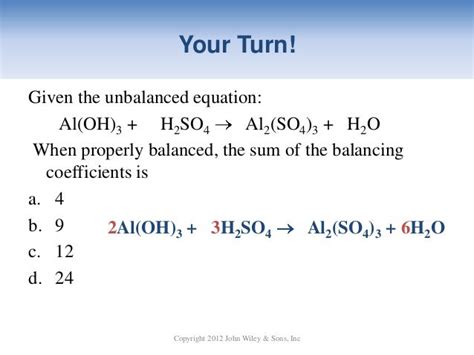
Table of Contents
Al(OH)₃ + H₂SO₄: A Deep Dive into the Balanced Equation and its Implications
The reaction between aluminum hydroxide, Al(OH)₃, and sulfuric acid, H₂SO₄, is a classic example of an acid-base neutralization reaction. Understanding this reaction, including balancing its equation and exploring its implications, is crucial for various fields, from chemistry education to industrial applications. This article provides a comprehensive overview, exploring the balanced equation, the stoichiometry involved, the type of reaction, the products formed, and the practical significance of this neutralization process.
Understanding the Reactants: Al(OH)₃ and H₂SO₄
Before delving into the reaction itself, let's briefly examine the individual reactants: aluminum hydroxide and sulfuric acid.
Aluminum Hydroxide, Al(OH)₃
Aluminum hydroxide is an amphoteric compound, meaning it can act as both an acid and a base. It's a white, crystalline solid that's relatively insoluble in water. Its amphoteric nature allows it to react with both strong acids and strong bases. In this reaction, it acts as a base, accepting protons (H⁺) from the sulfuric acid. Its role in neutralization reactions is significant, particularly in applications requiring pH control.
Sulfuric Acid, H₂SO₄
Sulfuric acid is a strong, highly corrosive mineral acid. It's a diprotic acid, meaning it can donate two protons (H⁺) per molecule. This characteristic is vital in its reaction with aluminum hydroxide, leading to a more complex stoichiometry than a monoprotic acid would produce. Its widespread use in various industrial processes highlights its chemical importance.
Balancing the Equation: Al(OH)₃ + H₂SO₄ → ?
The reaction between aluminum hydroxide and sulfuric acid produces aluminum sulfate and water. The unbalanced equation is:
Al(OH)₃ + H₂SO₄ → Al₂(SO₄)₃ + H₂O
Balancing this equation requires ensuring that the number of atoms of each element is equal on both sides of the equation. This is achieved by adjusting the stoichiometric coefficients:
2Al(OH)₃ + 3H₂SO₄ → Al₂(SO₄)₃ + 6H₂O
This balanced equation shows that two moles of aluminum hydroxide react with three moles of sulfuric acid to produce one mole of aluminum sulfate and six moles of water. This stoichiometric ratio is crucial for calculating the quantities of reactants needed or products produced in a given reaction. Understanding this ratio is fundamental to quantitative chemical analysis.
Stoichiometry and Calculations
The balanced equation provides the basis for performing stoichiometric calculations. For instance, if we know the amount of aluminum hydroxide used, we can calculate the amount of sulfuric acid required for complete neutralization or the amount of aluminum sulfate and water produced. Conversely, if we know the amount of sulfuric acid used, we can determine the amount of aluminum hydroxide needed and the resulting products. These calculations are essential in laboratory settings and industrial processes to ensure efficient and controlled reactions.
Example Calculation
Let's say we have 10 moles of Al(OH)₃. Using the balanced equation:
2Al(OH)₃ + 3H₂SO₄ → Al₂(SO₄)₃ + 6H₂O
We can determine the moles of H₂SO₄ required:
(10 moles Al(OH)₃) * (3 moles H₂SO₄ / 2 moles Al(OH)₃) = 15 moles H₂SO₄
Therefore, 15 moles of sulfuric acid are needed to completely react with 10 moles of aluminum hydroxide. Similarly, we can calculate the moles of Al₂(SO₄)₃ and H₂O produced. This demonstrates the practical application of the balanced equation in quantitative chemistry.
The Nature of the Reaction: Acid-Base Neutralization
The reaction between aluminum hydroxide and sulfuric acid is a classic example of an acid-base neutralization reaction. In this reaction, the hydroxide ions (OH⁻) from the aluminum hydroxide react with the hydrogen ions (H⁺) from the sulfuric acid to form water. This process neutralizes the acidic properties of the sulfuric acid, resulting in a less acidic or even neutral solution, depending on the stoichiometry of the reactants. The neutralization reaction is an exothermic process, meaning it releases heat.
Products of the Reaction: Al₂(SO₄)₃ and H₂O
The products of the reaction are aluminum sulfate, Al₂(SO₄)₃, and water, H₂O.
Aluminum Sulfate, Al₂(SO₄)₃
Aluminum sulfate is a water-soluble salt commonly used in various applications, including water treatment, as a flocculant to remove suspended particles. Its use in water purification highlights the practical significance of the aluminum hydroxide and sulfuric acid neutralization reaction. The properties of aluminum sulfate, such as its solubility and its ability to form precipitates, are important considerations in its various applications.
Water, H₂O
Water is the other product of the reaction. The formation of water is a key indicator of an acid-base neutralization reaction. The production of water further emphasizes the transfer of protons from the acid to the base, resulting in the neutralization of the solution's acidity.
Practical Applications and Significance
The reaction between aluminum hydroxide and sulfuric acid has various practical applications across diverse fields:
-
Water Treatment: As mentioned earlier, aluminum sulfate, a product of this reaction, is widely used as a coagulant or flocculant in water treatment plants. It helps in removing impurities and clarifying water.
-
Industrial Processes: Sulfuric acid is a crucial component in many industrial processes. Understanding its reaction with aluminum hydroxide is vital for controlling pH levels and managing waste streams in these industries.
-
Chemical Synthesis: This reaction serves as a fundamental step in several chemical syntheses, where aluminum sulfate or controlled pH conditions are required.
-
Laboratory Settings: The reaction is frequently demonstrated and studied in chemistry laboratories to illustrate acid-base neutralization reactions and stoichiometric calculations.
Beyond the Basics: Considering Reaction Conditions and Kinetics
While the balanced equation provides a fundamental understanding of the reaction, several other factors can influence its course:
-
Concentration of Reactants: The concentrations of aluminum hydroxide and sulfuric acid significantly affect the reaction rate and the final pH of the solution. Higher concentrations generally lead to faster reactions.
-
Temperature: Like most chemical reactions, the reaction rate increases with increasing temperature. Higher temperatures provide more kinetic energy, leading to more frequent and successful collisions between reactant molecules.
-
Presence of Catalysts: While not typically required, catalysts might influence the reaction rate under specific conditions.
-
Reaction Kinetics: A detailed kinetic study would involve exploring the reaction order, rate constant, and activation energy. These parameters provide a deeper understanding of the reaction's mechanism and its dependence on various factors.
Conclusion: A Fundamental Reaction with Broad Implications
The reaction between aluminum hydroxide and sulfuric acid, represented by the balanced equation 2Al(OH)₃ + 3H₂SO₄ → Al₂(SO₄)₃ + 6H₂O, is a fundamental acid-base neutralization reaction with significant practical implications. Understanding the stoichiometry, the nature of the reaction, the products formed, and the various factors that can influence its course is crucial for anyone working in chemistry-related fields, from students to industrial chemists. This reaction serves as a cornerstone in illustrating basic chemical principles and showcasing the practical applications of chemical reactions in real-world scenarios. The detailed understanding of this seemingly simple reaction opens doors to a broader understanding of more complex chemical processes.
Latest Posts
Latest Posts
-
What Is The Heart Of Computer
Mar 28, 2025
-
How Many Hours Does A Cow Sleep
Mar 28, 2025
-
30 Is 60 Of What Number
Mar 28, 2025
-
Complete The Following Table Using The Periodic Table
Mar 28, 2025
-
What Is The Major Product From The Following Reaction
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Al Oh 3 H2so4 Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
