A Single Chlorine Atom Can Destroy How Many Ozone Molecules
News Leon
Mar 31, 2025 · 5 min read
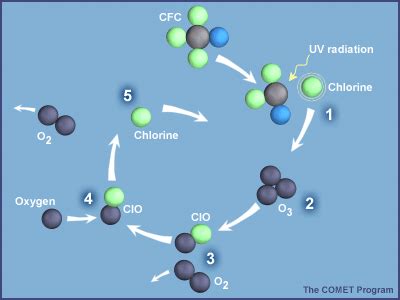
Table of Contents
A Single Chlorine Atom Can Destroy How Many Ozone Molecules? The Devastating Impact of CFCs
The depletion of the ozone layer is one of the most pressing environmental issues of our time. A crucial aspect of understanding this problem lies in comprehending the catalytic destruction of ozone molecules by single chlorine atoms, originating primarily from the breakdown of chlorofluorocarbons (CFCs). This article delves deep into the chemistry behind this destructive process, exploring the scale of the damage inflicted by a single chlorine atom and the far-reaching consequences for our planet.
The Chemistry of Ozone Depletion: A Chain Reaction
The ozone layer, residing in the stratosphere, plays a vital role in shielding Earth from harmful ultraviolet (UV) radiation from the sun. Ozone (O₃) is formed naturally through a complex series of reactions involving oxygen molecules (O₂) and UV radiation. However, this natural balance is disrupted by the presence of chlorine atoms, primarily released from the breakdown of CFCs.
CFCs, once widely used in refrigerants, aerosols, and other applications, are remarkably stable in the lower atmosphere. However, they eventually reach the stratosphere where intense UV radiation breaks them down, releasing chlorine atoms (Cl). This seemingly insignificant chlorine atom then initiates a devastating chain reaction.
The Catalytic Cycle: A Single Atom's Devastating Impact
A single chlorine atom doesn't simply react with a single ozone molecule and then become inert. Instead, it acts as a catalyst, participating in a cyclical process that can destroy thousands of ozone molecules before it's finally removed from the stratosphere. The process unfolds as follows:
-
Cl + O₃ → ClO + O₂: The chlorine atom (Cl) reacts with an ozone molecule (O₃), breaking it down into a chlorine monoxide molecule (ClO) and an oxygen molecule (O₂). This is the crucial initial step.
-
ClO + O → Cl + O₂: The chlorine monoxide (ClO) then reacts with a free oxygen atom (O), a naturally occurring component of the stratosphere. This reaction regenerates the chlorine atom (Cl) and produces another oxygen molecule (O₂).
-
Cycle Repetition: The regenerated chlorine atom (Cl) is now free to repeat the entire process, reacting with another ozone molecule and initiating another cycle of destruction. This catalytic cycle can continue for many years, with a single chlorine atom potentially destroying tens of thousands of ozone molecules before it is eventually removed from the stratosphere through other chemical reactions, such as the formation of chlorine nitrate (ClONO₂) or its reaction with methane (CH₄).
Estimating the Number of Ozone Molecules Destroyed: A Complex Calculation
Determining the precise number of ozone molecules destroyed by a single chlorine atom is difficult and depends on several factors:
-
Atmospheric Conditions: Temperature, pressure, and the concentration of other atmospheric constituents (such as oxygen atoms, methane, and other radicals) all influence the rate of the catalytic cycle and the lifetime of chlorine atoms in the stratosphere.
-
Reaction Rates: The rate constants for the individual reactions in the catalytic cycle vary with temperature, further complicating accurate calculations.
-
Removal Mechanisms: The efficiency of mechanisms that remove chlorine atoms from the catalytic cycle (e.g., formation of ClONO₂ or reaction with methane) determines the overall duration of the catalytic activity.
While a precise number is challenging to provide, scientific studies and models suggest that a single chlorine atom can, on average, destroy tens of thousands, even potentially hundreds of thousands, of ozone molecules before it is eventually removed from the stratosphere. This remarkable efficiency highlights the disproportionate impact of CFCs on the ozone layer.
The Long-Term Consequences: Beyond Ozone Depletion
The destruction of ozone molecules has far-reaching consequences, extending beyond simply thinning the ozone layer. Increased UV radiation reaching the Earth's surface leads to:
-
Increased Skin Cancer Rates: UV radiation is a major cause of skin cancer, and increased exposure due to ozone depletion significantly increases the risk of this deadly disease.
-
Eye Damage: UV radiation can damage the eyes, leading to cataracts and other vision problems.
-
Immune System Suppression: UV radiation can suppress the immune system, making individuals more susceptible to infections.
-
Damage to Plants and Ecosystems: UV radiation can harm plant life, impacting agricultural yields and disrupting entire ecosystems.
The Montreal Protocol: A Success Story in Environmental Protection
Recognizing the severity of ozone depletion, the international community adopted the Montreal Protocol on Substances that Deplete the Ozone Layer in 1987. This landmark agreement phased out the production and consumption of CFCs and other ozone-depleting substances (ODS).
The Montreal Protocol has been hailed as a remarkable success story in international environmental cooperation. The concentration of CFCs in the atmosphere has begun to decline, and the ozone layer is showing signs of recovery. However, the long lifetime of CFCs in the atmosphere means that the full recovery of the ozone layer will take several decades.
Ongoing Research and Monitoring: Ensuring Continued Recovery
Despite the success of the Montreal Protocol, ongoing research and monitoring are crucial to ensure the continued recovery of the ozone layer. Scientists continue to study the atmospheric chemistry of ozone depletion, develop improved models to predict future ozone levels, and monitor the effectiveness of the phase-out of ODS. Furthermore, research focuses on identifying and regulating new potential ozone-depleting substances.
Conclusion: The Importance of Understanding Ozone Depletion
The catalytic destruction of ozone molecules by chlorine atoms, originating from CFCs, serves as a stark reminder of the profound impact human activities can have on the environment. Understanding the scale of damage inflicted by a single chlorine atom is essential for appreciating the urgency and effectiveness of international cooperation like the Montreal Protocol. While the ozone layer is recovering, ongoing vigilance, research, and adherence to international agreements remain critical to ensure its complete restoration and protect our planet from the harmful effects of increased UV radiation. The story of ozone depletion stands as a powerful testament to both the destructive power of human-made chemicals and the potential for international collaboration to address global environmental challenges. The lesson learned – a single atom's devastating power – underscores the need for continued vigilance and sustainable practices in the future.
Latest Posts
Latest Posts
-
Frozen Orange Juice Is Reconstituted By Adding Water To It
Apr 02, 2025
-
Draw And Label One Complete Cell Cycle
Apr 02, 2025
-
A Physical Combination Of Two Or More Substances
Apr 02, 2025
-
What Is The Difference Between A Primary And Secondary Consumer
Apr 02, 2025
-
Which One Of The Following Is An Igneous Rock
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about A Single Chlorine Atom Can Destroy How Many Ozone Molecules . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
