A Semipermeable Membrane Is Placed Between
News Leon
Apr 01, 2025 · 6 min read
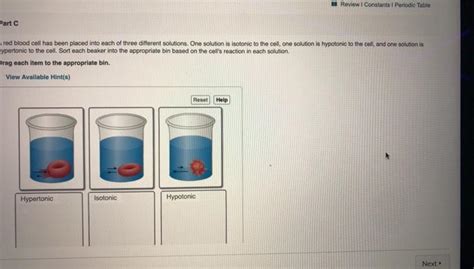
Table of Contents
A Semipermeable Membrane is Placed Between: Exploring Osmosis and Diffusion
A semipermeable membrane, a selective barrier allowing only certain molecules or ions to pass through, is a fundamental concept in biology and chemistry. Placing a semipermeable membrane between two solutions initiates processes like osmosis and diffusion, shaping various biological phenomena and impacting numerous industrial applications. This detailed exploration dives into these processes, their mechanisms, and their far-reaching consequences.
Understanding Semipermeable Membranes
A semipermeable membrane, also known as a selectively permeable membrane, acts as a gatekeeper, controlling the movement of substances. Its selectivity stems from its molecular structure, which features pores or channels of specific sizes and properties. Small molecules like water and certain gases can easily pass through, while larger molecules or ions are blocked. The exact permeability depends on the membrane's material; examples include cell membranes (phospholipid bilayers), dialysis tubing, and specialized synthetic membranes.
Types of Semipermeable Membranes
Several types of semipermeable membranes exist, each tailored for specific applications:
-
Biological Membranes: Found in all living cells, these membranes are primarily composed of phospholipids arranged in a bilayer, interspersed with proteins and cholesterol. Their selective permeability is crucial for maintaining cellular homeostasis and enabling various cellular processes.
-
Synthetic Membranes: These artificial membranes are designed for specific applications, such as water purification, dialysis, and gas separation. They are often made from polymers like cellulose acetate or polysulfone and can be engineered to have specific pore sizes and functionalities.
-
Dialysis Membranes: Specifically designed for dialysis treatments, these membranes are highly permeable to small waste molecules like urea and creatinine but retain larger proteins and blood cells.
Osmosis: The Movement of Water Across a Membrane
Osmosis is a specific type of passive transport where water molecules move across a semipermeable membrane from a region of higher water concentration (lower solute concentration) to a region of lower water concentration (higher solute concentration). This movement continues until equilibrium is reached, meaning the water concentration is equal on both sides of the membrane.
Osmotic Pressure
The pressure required to prevent the net movement of water across a semipermeable membrane is known as osmotic pressure. This pressure is directly proportional to the concentration of the solute; higher solute concentration leads to higher osmotic pressure. Understanding osmotic pressure is crucial in various applications, including intravenous fluid administration and plant water uptake.
Tonicity and Cell Behavior
The concept of tonicity describes the relative concentration of solutes in two solutions separated by a semipermeable membrane. It affects how cells behave in different solutions:
-
Isotonic Solution: The solute concentration is equal inside and outside the cell. Water moves in and out at equal rates, maintaining cell volume.
-
Hypotonic Solution: The solute concentration is lower outside the cell than inside. Water moves into the cell, causing it to swell and potentially lyse (burst).
-
Hypertonic Solution: The solute concentration is higher outside the cell than inside. Water moves out of the cell, causing it to shrink or crenate.
These effects of tonicity are critical for maintaining the health and function of cells. For example, red blood cells must be kept in an isotonic solution to prevent damage.
Diffusion: The Movement of Solutes Across a Membrane
Diffusion is the net movement of molecules or ions from a region of higher concentration to a region of lower concentration. This movement continues until the concentration is uniform throughout the system. Unlike osmosis, which specifically refers to water movement, diffusion can involve any type of molecule or ion.
Factors Affecting Diffusion Rate
Several factors influence the rate of diffusion:
-
Concentration Gradient: A steeper concentration gradient leads to faster diffusion.
-
Temperature: Higher temperatures increase the kinetic energy of molecules, accelerating diffusion.
-
Molecular Size: Smaller molecules diffuse faster than larger molecules.
-
Membrane Permeability: The permeability of the membrane to the diffusing substance plays a significant role. A more permeable membrane allows for faster diffusion.
Facilitated Diffusion
Some molecules, even if small, cannot readily diffuse across a membrane due to their polarity or other properties. Facilitated diffusion involves the use of membrane proteins to assist the movement of these molecules across the membrane. These proteins act as channels or carriers, providing a pathway for specific molecules to pass through. This process remains passive; it doesn't require energy input.
Osmosis and Diffusion in Biological Systems
Osmosis and diffusion are essential for numerous biological processes:
Cell Membrane Transport
The cell membrane acts as a semipermeable barrier, controlling the passage of substances in and out of the cell. Both osmosis and diffusion are crucial for maintaining cellular homeostasis, nutrient uptake, and waste removal.
Nutrient Absorption
In the digestive system, nutrients are absorbed from the gut lumen into the bloodstream through diffusion and osmosis. The concentration gradients established across the intestinal lining drive the uptake of essential molecules.
Water Regulation
Osmosis plays a vital role in regulating water balance in the body. The kidneys control the amount of water reabsorbed into the bloodstream, maintaining appropriate blood volume and electrolyte balance. This is achieved through controlled water movement across semipermeable membranes in the nephrons.
Plant Water Uptake
Plants absorb water from the soil through osmosis. Water moves from the soil (higher water potential) into the roots (lower water potential), driven by the osmotic pressure gradient. This process is crucial for plant growth and survival.
Applications of Semipermeable Membranes
Beyond their biological significance, semipermeable membranes find widespread applications in various industries:
Water Purification
Reverse osmosis uses pressure to force water through a semipermeable membrane, removing impurities like salts and other dissolved solids. This technology is widely used for producing potable water and purifying wastewater.
Dialysis
Dialysis employs semipermeable membranes to remove waste products from the blood of patients with kidney failure. The membrane allows small waste molecules to pass through while retaining larger proteins and blood cells.
Gas Separation
Semipermeable membranes are used to separate different gases from gas mixtures. This has applications in various industries, including air separation and natural gas processing.
Drug Delivery
Some drug delivery systems utilize semipermeable membranes to control the release of medication over time. This allows for sustained drug levels, reducing the frequency of administration and potentially minimizing side effects.
Future Directions
Research into semipermeable membranes continues to advance, exploring novel materials and applications. This includes the development of more efficient and durable membranes for water purification, improved dialysis techniques, and innovative drug delivery systems. Furthermore, advancements in nanotechnology are paving the way for creating membranes with even more precise control over permeability and functionality. The development of biomimetic membranes, mimicking the sophisticated properties of biological membranes, is another exciting area of research. These membranes could revolutionize various fields, including medicine, biotechnology, and environmental remediation.
Conclusion
The placement of a semipermeable membrane between two solutions initiates a dynamic interplay between osmosis and diffusion, shaping biological processes and impacting countless applications. Understanding these processes and the properties of semipermeable membranes is crucial for advancing scientific knowledge and developing innovative technologies. Further research promises to uncover new possibilities and broaden the scope of this essential scientific concept. From sustaining life at the cellular level to purifying water on a large scale, the impact of semipermeable membranes remains profound and far-reaching. Continued exploration of their properties and functionalities will undoubtedly lead to further breakthroughs in the years to come.
Latest Posts
Latest Posts
-
Name The Region That Attaches Two Sister Chromatids
Apr 02, 2025
-
How Many Grams Are In 5 66 Mol Of Caco3
Apr 02, 2025
-
What Is 13 25 As A Decimal
Apr 02, 2025
-
How Many Months Are In Five Years
Apr 02, 2025
-
Geometric Mean Of 9 And 4
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about A Semipermeable Membrane Is Placed Between . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
