A Pure Substance Containing Two Or More
News Leon
Mar 14, 2025 · 6 min read
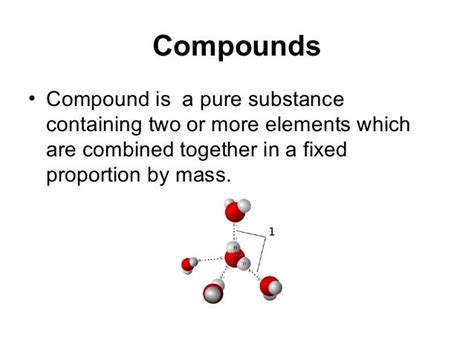
Table of Contents
A Pure Substance Containing Two or More: Delving into the World of Mixtures
A pure substance, by definition, is a form of matter that has a constant composition and properties throughout the sample. This means that it's made up of only one type of atom or molecule. However, the statement "a pure substance containing two or more" seems contradictory at first glance. The key lies in understanding the distinction between pure substances and mixtures. While a pure substance is comprised of only one type of particle, a mixture is a combination of two or more pure substances that are physically combined but not chemically bonded. This seemingly paradoxical phrase, therefore, refers to the components within a mixture, each of which is a pure substance in itself. Let's delve deeper into this fascinating concept.
Understanding Pure Substances
Before examining mixtures, it's crucial to solidify our understanding of pure substances. These are materials with a uniform composition and consistent properties. They can be further categorized into:
1. Elements:
Elements are the fundamental building blocks of matter. They are substances that cannot be broken down into simpler substances by chemical means. Each element is characterized by its unique atomic number, representing the number of protons in its nucleus. Examples include oxygen (O), hydrogen (H), iron (Fe), and gold (Au). These are pure substances because each atom within a sample of an element is identical.
2. Compounds:
Compounds are pure substances formed by the chemical combination of two or more elements in fixed proportions. This combination involves the formation of chemical bonds, creating a new substance with properties distinct from its constituent elements. Water (H₂O), for example, is a compound formed from the chemical bonding of two hydrogen atoms and one oxygen atom. Table salt (NaCl), or sodium chloride, is another example, a compound formed from sodium and chlorine. The ratio of elements in a compound is always constant and defined by its chemical formula.
Exploring Mixtures: A Blend of Pure Substances
A mixture, unlike a compound, is a physical combination of two or more pure substances. The components of a mixture retain their individual chemical properties and are not chemically bonded. Mixtures can be separated into their constituent components by physical methods, such as filtration, distillation, evaporation, or chromatography.
Mixtures are broadly classified into:
1. Homogeneous Mixtures (Solutions):
In homogeneous mixtures, the components are uniformly distributed throughout the sample. This means that the composition is the same throughout, and you can't visually distinguish the individual components. Examples include saltwater (salt dissolved in water), air (a mixture of various gases like nitrogen, oxygen, and carbon dioxide), and sugar dissolved in water. The key characteristic is uniformity at the macroscopic level.
Examples of Homogeneous Mixtures Containing Multiple Pure Substances:
- Brass: An alloy of copper and zinc, brass is a homogeneous mixture where the copper and zinc atoms are uniformly distributed.
- Steel: A mixture of iron and carbon (with trace amounts of other elements), steel presents a homogeneous structure at a macroscopic level.
- Air: As mentioned before, air is a homogeneous mixture of gases, each a pure substance in itself.
2. Heterogeneous Mixtures:
Heterogeneous mixtures have a non-uniform composition. The components are not evenly distributed, and you can visually distinguish the different parts of the mixture. Examples include sand and water, oil and water, and a salad. In these mixtures, distinct regions or phases with different compositions are observable.
Examples of Heterogeneous Mixtures Containing Multiple Pure Substances:
- Granite: Granite is a rock composed of different minerals like quartz, feldspar, and mica. Each mineral is a pure substance, but their distribution within granite is heterogeneous.
- Soil: Soil is a complex heterogeneous mixture containing various minerals, organic matter, water, and air. Each constituent is a pure substance, but they are not uniformly distributed.
- Concrete: Concrete is a mixture of cement, aggregates (sand and gravel), and water. While it appears solid, its composition is not homogeneous at a microscopic level.
The Significance of the Distinction
The distinction between pure substances and mixtures is crucial in various fields:
- Chemistry: Understanding the composition of substances is fundamental to chemical reactions and analysis. Pure substances have predictable properties, while mixtures can exhibit variable properties depending on the proportions of their components.
- Materials Science: The properties of materials are often determined by their composition and structure. The design of alloys and composite materials depends on carefully controlling the mixture of pure substances.
- Environmental Science: The analysis of pollutants and environmental samples requires identifying both pure substances and mixtures. Understanding the composition of mixtures is crucial for assessing environmental quality and developing remediation strategies.
- Pharmaceuticals: The purity of pharmaceutical ingredients is vital for safety and efficacy. Impurities in mixtures can drastically alter the properties of a drug.
- Food Science: Understanding the composition of food mixtures is essential for quality control, processing, and nutrition. The mix of ingredients dictates the overall nutritional profile and sensory properties.
Separating Mixtures: Techniques and Applications
Because mixtures are physically combined, they can be separated into their constituent pure substances through various physical methods. The choice of technique depends on the properties of the components in the mixture.
1. Filtration:
Filtration separates solids from liquids using a porous material, like filter paper. The liquid passes through the filter, leaving the solid behind. This is commonly used to separate sand from water or to remove impurities from a liquid.
2. Distillation:
Distillation separates liquids based on their boiling points. The mixture is heated, and the component with the lowest boiling point vaporizes first, which is then condensed and collected. This process is crucial for purifying water or separating components of crude oil.
3. Evaporation:
Evaporation separates a dissolved solid from a liquid by allowing the liquid to evaporate, leaving the solid behind. This is commonly used to obtain salt from saltwater.
4. Chromatography:
Chromatography separates components of a mixture based on their differing affinities for a stationary phase and a mobile phase. This technique is highly versatile and is used to separate complex mixtures, such as pigments in ink or compounds in a biological sample.
5. Magnetism:
This method separates magnetic materials from non-magnetic materials. A magnet is used to attract magnetic substances like iron filings, leaving behind non-magnetic materials.
Conclusion: The Interplay of Purity and Mixture
The concept of "a pure substance containing two or more" highlights the vital distinction between pure substances and mixtures. While seemingly contradictory, it emphasizes that mixtures are composed of individual pure substances that retain their chemical identity even within the mixture. Understanding this interplay is paramount in various scientific fields, allowing us to analyze, manipulate, and utilize materials effectively. From the development of advanced materials to the purification of pharmaceuticals, the ability to separate and analyze mixtures is instrumental in numerous technological advancements and scientific discoveries. The diverse techniques for separating mixtures further underscore the importance of this fundamental concept in chemistry and beyond. The study of mixtures allows for a deeper comprehension of the physical and chemical properties of matter, enabling us to create and innovate in countless ways.
Latest Posts
Latest Posts
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about A Pure Substance Containing Two Or More . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
