A Mixture Of N2 And H2 Is Caused To React
News Leon
Mar 27, 2025 · 5 min read
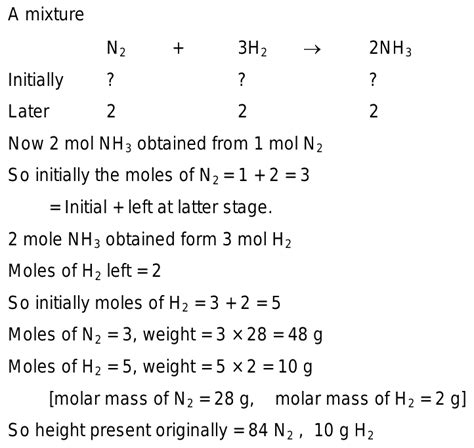
Table of Contents
A Mixture of N₂ and H₂ is Caused to React: Exploring the Haber-Bosch Process and Beyond
The reaction between nitrogen (N₂) and hydrogen (H₂) to produce ammonia (NH₃) is a cornerstone of modern industrial chemistry. This seemingly simple reaction, however, holds a fascinating depth, encompassing intricate chemical kinetics, significant industrial applications, and ongoing research into optimizing its efficiency. This article delves into the details of this crucial reaction, exploring the Haber-Bosch process, its underlying principles, challenges, and future prospects.
The Haber-Bosch Process: A Cornerstone of Modern Agriculture
The Haber-Bosch process is the industrial method used to synthesize ammonia on a massive scale. Developed in the early 20th century by Fritz Haber and Carl Bosch, it revolutionized agriculture by providing a readily available source of nitrogen fertilizer. Before the Haber-Bosch process, nitrogen-based fertilizers were limited, restricting agricultural yields and impacting global food security.
The Reaction:
The core of the Haber-Bosch process is the following reversible exothermic reaction:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
This reaction, while seemingly straightforward, presents several significant challenges that have driven decades of research and innovation.
Key Challenges in the Haber-Bosch Process
1. Kinetic Limitations: The reaction between nitrogen and hydrogen is inherently slow at ambient temperatures due to the strong triple bond in the nitrogen molecule (N₂). This requires high temperatures to overcome the activation energy barrier.
2. Thermodynamic Equilibrium: The reaction is exothermic, meaning it releases heat. According to Le Chatelier's principle, increasing the temperature shifts the equilibrium towards the reactants (N₂ and H₂), reducing the yield of ammonia. Therefore, a compromise must be reached between temperature and yield.
3. Pressure Optimization: Increasing the pressure favors the formation of ammonia, as the reaction results in a decrease in the number of gas molecules (4 moles of reactants produce 2 moles of product). However, extremely high pressures require robust and expensive equipment.
4. Catalyst Selection: The use of a catalyst is crucial to accelerate the reaction rate and lower the activation energy. Historically, iron-based catalysts have been used extensively, although research is continually focused on improving their activity and selectivity. Promoters, such as potassium and aluminum oxides, are added to enhance catalyst performance.
Optimizing the Haber-Bosch Process: A Balancing Act
The successful industrial implementation of the Haber-Bosch process involves carefully balancing the competing factors mentioned above. Optimal conditions typically involve:
- Temperature: Around 450-500 °C. While higher temperatures speed up the reaction, they reduce the equilibrium yield.
- Pressure: Around 200-250 atm. High pressure favors the product, but increases equipment costs and energy consumption.
- Catalyst: A finely divided iron catalyst, often with promoters, plays a vital role in achieving a reasonable rate of reaction.
Beyond the Basics: Advanced Considerations
The Haber-Bosch process isn't simply a matter of mixing nitrogen and hydrogen under specific conditions. Several other intricate aspects require careful management:
1. Feedstock Purification: Highly pure nitrogen and hydrogen are essential to prevent catalyst poisoning and maintain optimal reaction efficiency. Impurities can significantly impact catalyst activity and longevity.
2. Gas Recycling: Due to the equilibrium nature of the reaction, only a fraction of the reactants is converted to ammonia in a single pass. Unreacted gases are recycled back into the reactor to enhance overall ammonia yield.
3. Heat Management: The exothermic nature of the reaction necessitates efficient heat removal to maintain the desired temperature and prevent overheating. This often involves sophisticated heat exchange systems.
4. Process Monitoring and Control: Maintaining precise control over temperature, pressure, and gas flow is crucial for optimizing ammonia production and ensuring process safety. Modern Haber-Bosch plants utilize advanced instrumentation and automation systems.
The Environmental Impact: A Double-Edged Sword
The Haber-Bosch process has undoubtedly revolutionized agriculture, but it comes with environmental consequences:
- Energy Consumption: The process is energy-intensive, requiring vast amounts of natural gas or other fossil fuels for heat and power generation. This contributes significantly to greenhouse gas emissions.
- Greenhouse Gas Emissions: Besides direct energy consumption, the production of hydrogen often involves steam methane reforming, which releases carbon dioxide. Ammonia production is thus indirectly linked to significant CO₂ emissions.
- Water Consumption: The production of hydrogen, and consequently ammonia, requires significant amounts of water. This can strain water resources in certain regions.
Research and Development: Toward a Greener Future
Recognizing the significant environmental impact of the Haber-Bosch process, research is actively focused on developing more sustainable alternatives:
- Renewable Energy Sources: Exploring the use of renewable energy sources, such as solar and wind power, to drive the process could significantly reduce greenhouse gas emissions.
- Electrochemical Synthesis: Electrochemical methods offer a potential route to ammonia synthesis using renewable electricity to split water and produce hydrogen, circumventing the need for fossil fuels.
- Catalyst Optimization: Ongoing research aims to develop more efficient and selective catalysts, potentially reducing the energy requirements and improving ammonia yield.
- Alternative Nitrogen Sources: Investigating alternative sources of nitrogen, such as captured nitrogen oxides from industrial emissions, could potentially reduce reliance on atmospheric nitrogen.
The Future of Ammonia Synthesis: Meeting Global Challenges
The demand for ammonia, primarily for fertilizers, continues to rise alongside the global population. Addressing the environmental challenges associated with the Haber-Bosch process is crucial for sustainable food production and environmental stewardship. Future developments in this field are likely to focus on:
- Decentralized Ammonia Production: Smaller-scale, localized ammonia production units powered by renewable energy could reduce transportation costs and environmental impact.
- Improved Process Integration: Integrating ammonia production with other industrial processes, such as hydrogen production or carbon capture, could enhance efficiency and sustainability.
- Advanced Process Control and Optimization: Utilizing advanced process control techniques, machine learning, and artificial intelligence could optimize reaction parameters and improve overall process efficiency.
Conclusion: A Continuing Legacy
The reaction between nitrogen and hydrogen, implemented through the Haber-Bosch process, remains a pivotal technology in modern society. While its environmental impact necessitates ongoing research and development efforts, the process’s significance in ensuring global food security is undeniable. The pursuit of a greener, more sustainable approach to ammonia synthesis is not only crucial for environmental protection but also essential for ensuring the long-term sustainability of food production and meeting the demands of a growing global population. The journey towards a more efficient and environmentally friendly ammonia production process is a testament to the ongoing evolution of chemical engineering and its role in shaping a sustainable future. The future of ammonia synthesis will undoubtedly involve a combination of technological advancements, policy changes, and a renewed focus on resource efficiency and environmental responsibility.
Latest Posts
Latest Posts
-
Why Are Sex Linked Traits More Common In Males Than Females
Mar 30, 2025
-
Difference Between Specific Gravity And Density
Mar 30, 2025
-
Why Did The Pony Express Only Last 18 Months
Mar 30, 2025
-
Which Of The Following Is A True Statement About Vitamins
Mar 30, 2025
-
What Sea Separates Europe From Africa
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about A Mixture Of N2 And H2 Is Caused To React . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
