A Catalyst Increases The Rate Of A Chemical Reaction By
News Leon
Mar 22, 2025 · 5 min read
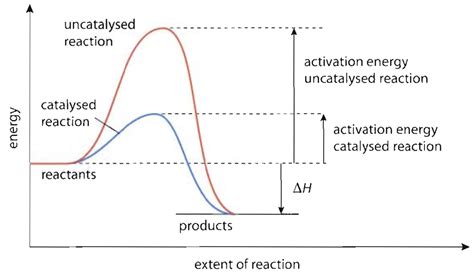
Table of Contents
A Catalyst Increases the Rate of a Chemical Reaction By… Lowering the Activation Energy
A catalyst is a substance that increases the rate of a chemical reaction without itself being consumed in the process. This seemingly magical ability is fundamental to countless industrial processes, biological functions, and even everyday occurrences. But how does a catalyst achieve this speed boost? The answer lies in its ability to lower the activation energy of a reaction.
Understanding Activation Energy: The Energy Barrier
Imagine a chemical reaction as two mountains separated by a high valley. To get from one mountain (reactants) to the other (products), you need to climb over the valley – this represents the activation energy (Ea). This energy is the minimum amount of energy required for the reactant molecules to collide with sufficient force and correct orientation to break existing bonds and form new ones, leading to product formation. Reactions with high activation energies proceed slowly because only a small fraction of molecules possess the necessary energy at a given temperature.
The Role of Collisions and Orientation
The success of a chemical reaction hinges on two critical factors during molecular collisions: sufficient energy and correct orientation. Even if molecules possess enough kinetic energy, an ineffective collision due to poor orientation will not lead to a reaction. The activation energy encompasses both these factors, representing the energy needed to overcome the energetic and geometric barriers to reaction.
How Catalysts Lower Activation Energy: Alternative Pathways
Catalysts don't magically add energy to the system; instead, they provide an alternative reaction pathway with a lower activation energy. This new pathway allows the reaction to proceed faster because a larger fraction of molecules now possess the energy required to traverse this lower energy barrier. This is analogous to building a tunnel through the valley separating our mountains; the tunnel bypasses the high climb, making the journey significantly easier and faster.
Different Types of Catalysis: Homogeneous and Heterogeneous
Catalysts are broadly classified into two categories:
-
Homogeneous Catalysts: These catalysts exist in the same phase (solid, liquid, or gas) as the reactants. For example, the acidic catalysis of esterification reactions, where a strong acid like sulfuric acid acts as a homogeneous catalyst in a liquid phase reaction. The catalyst is intimately mixed with the reactants, facilitating close interaction and efficient catalysis.
-
Heterogeneous Catalysts: These catalysts exist in a different phase from the reactants. A classic example is the use of platinum in catalytic converters in automobiles, where the platinum (solid) catalyzes the conversion of harmful gases (gases) into less harmful products. Heterogeneous catalysis often involves adsorption of reactants onto the catalyst surface, where the reaction takes place before the products desorb.
Mechanism of Catalysis: A Detailed Look
The precise mechanism by which a catalyst lowers activation energy depends on the specific reaction and catalyst. However, some common mechanisms include:
-
Formation of Intermediate Complexes: The catalyst interacts with the reactants to form intermediate complexes. These complexes have lower activation energies for subsequent steps in the reaction, leading to overall acceleration.
-
Orientation and Proximity Effects: The catalyst brings reactant molecules closer together and orients them favorably, increasing the probability of successful collisions. This is particularly relevant in heterogeneous catalysis, where reactants adsorb onto the catalyst surface in a specific orientation, thereby facilitating bond breaking and formation.
-
Electron Transfer: Some catalysts participate in electron transfer processes, facilitating bond breaking or formation by altering the electronic structure of the reactants. This is common in redox reactions.
-
Acid-Base Catalysis: In acid-base catalysis, the catalyst (acid or base) donates or accepts a proton, altering the reactivity of the reactant molecule and lowering the activation energy.
Examples of Catalysis in Action
Catalysis is pervasive in various aspects of our lives:
-
Enzymes in Biological Systems: Enzymes are biological catalysts that accelerate virtually all biochemical reactions in living organisms. Their highly specific nature ensures efficient and controlled metabolic processes. They are highly efficient and operate under mild conditions.
-
Industrial Processes: The Haber-Bosch process for ammonia synthesis relies on an iron catalyst to achieve high yields. Similarly, numerous other industrial processes, such as the production of plastics, pharmaceuticals, and fuels, depend on catalysts for efficiency and economic viability.
-
Catalytic Converters in Automobiles: These devices use platinum, palladium, and rhodium catalysts to convert harmful exhaust gases (carbon monoxide, nitrogen oxides, and unburned hydrocarbons) into less harmful substances (carbon dioxide, nitrogen, and water).
-
Everyday Life: Even seemingly simple processes, like the ripening of fruit or the rusting of iron, are influenced by catalysts.
Factors Affecting Catalytic Activity
Several factors can influence the activity of a catalyst:
-
Temperature: Increasing temperature generally increases the rate of a catalyzed reaction, but excessive heat can also degrade the catalyst.
-
Catalyst Concentration: Higher catalyst concentration generally leads to a faster reaction rate, up to a point where saturation occurs.
-
Surface Area (for heterogeneous catalysts): A larger surface area provides more active sites for reactant interaction, leading to increased activity. This is why heterogeneous catalysts are often finely divided or porous.
-
Presence of Inhibitors or Poisons: Impurities or substances that bind strongly to the active sites of a catalyst can reduce its effectiveness, even completely deactivating it.
-
pH (for homogeneous catalysts): The pH of the reaction medium can influence the activity of acid or base catalysts.
Specificity and Selectivity: The Power of Catalysts
Catalysts are not just about speeding up reactions; they also exhibit remarkable specificity and selectivity. Specificity refers to a catalyst's ability to accelerate only a specific reaction, while selectivity means it can favor the formation of a particular product over others in a reaction with multiple possible outcomes. Enzymes are a prime example, catalyzing very specific reactions within the complex milieu of a living cell without interfering with other processes. This specificity and selectivity are crucial in achieving desired outcomes in both biological and industrial contexts.
Conclusion: The Indispensable Role of Catalysts
Catalysts play an indispensable role in countless chemical processes, driving efficiency, controlling selectivity, and enabling reactions that would otherwise be too slow or impossible under normal conditions. Their ability to lower activation energy lies at the heart of their effectiveness, making them fundamental tools in chemistry, biology, and numerous industrial applications. The ongoing research and development in catalyst design and optimization continue to unlock new possibilities and drive innovation across a wide range of fields. Understanding the principles behind catalytic activity is crucial to leveraging this powerful tool for technological advancement and a sustainable future.
Latest Posts
Latest Posts
-
Guanine Forms Hydrogen Bonds With Cytosine
Mar 23, 2025
-
A Quadrilateral Where Each Angle Is A Right Angle
Mar 23, 2025
-
Shape With 12 Edges And 6 Faces
Mar 23, 2025
-
48 Hours Is Equal To How Many Days
Mar 23, 2025
-
Is Fructose An Aldose Or Ketose
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about A Catalyst Increases The Rate Of A Chemical Reaction By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
