Guanine Forms Hydrogen Bonds With Cytosine
News Leon
Mar 23, 2025 · 6 min read
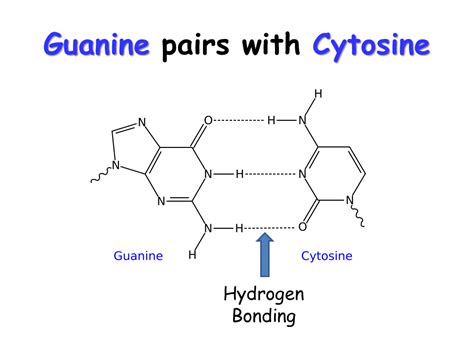
Table of Contents
Guanine Forms Hydrogen Bonds with Cytosine: A Deep Dive into Base Pairing
The fundamental building blocks of life, DNA and RNA, owe their remarkable properties to the precise pairing of their constituent nucleobases. Among these bases, the interaction between guanine (G) and cytosine (C) via hydrogen bonding stands as a cornerstone of molecular biology. This article delves into the intricacies of G-C base pairing, exploring its structural features, energetic considerations, and crucial role in the stability and function of nucleic acids.
Understanding the Players: Guanine and Cytosine
Before examining the hydrogen bonding itself, let's briefly review the structures of guanine and cytosine. Both are purine and pyrimidine bases, respectively, meaning they are heterocyclic aromatic compounds containing nitrogen atoms within their ring structures.
Guanine: A Purine Base
Guanine, a purine base with a double-ring structure, possesses a carbonyl group (=O) at position 6 and an amino group (-NH₂) at position 2. These functional groups play a crucial role in forming hydrogen bonds with cytosine. Its specific arrangement of atoms contributes to its compatibility with cytosine, making the G-C interaction highly specific and energetically favorable. The precise geometry of these groups is critical for ensuring the correct spatial alignment for hydrogen bond formation.
Cytosine: A Pyrimidine Base
Cytosine, a pyrimidine base with a single-ring structure, features an amino group (-NH₂) at position 4 and a carbonyl group (=O) at position 2. These functional groups, analogous to those in guanine, are strategically positioned to interact with guanine's functional groups, forming the characteristic hydrogen bonds. The electron distribution within the cytosine ring is also a crucial factor in influencing its interaction with guanine, facilitating stable hydrogen bond formation.
The Hydrogen Bonds: The Glue of Life
The interaction between guanine and cytosine is characterized by the formation of three hydrogen bonds. This is in contrast to the two hydrogen bonds formed between adenine and thymine (or uracil in RNA). This difference in the number of hydrogen bonds significantly impacts the overall stability of the DNA double helix. Let's examine each hydrogen bond individually:
Hydrogen Bond 1: Guanine's Amino Group to Cytosine's Carbonyl Oxygen
The first hydrogen bond is formed between the amino group (-NH₂) at position 2 of guanine and the carbonyl oxygen (=O) at position 2 of cytosine. The hydrogen atom from the amino group acts as a hydrogen bond donor, while the oxygen atom of the carbonyl group acts as a hydrogen bond acceptor. This interaction is relatively strong due to the electronegativity difference between nitrogen and oxygen.
Hydrogen Bond 2: Guanine's Carbonyl Oxygen to Cytosine's Amino Hydrogen
The second hydrogen bond involves the carbonyl oxygen (=O) at position 6 of guanine and the amino hydrogen (-NH) at position 4 of cytosine. In this case, the oxygen of the guanine carbonyl acts as a hydrogen bond acceptor, and the hydrogen atom of the cytosine amino group acts as the hydrogen bond donor. This interaction contributes significantly to the overall strength of the G-C base pair.
Hydrogen Bond 3: Guanine's N1 to Cytosine's Amino Hydrogen
The third hydrogen bond, which enhances the stability of the G-C base pair even further, involves the nitrogen atom (N1) in the guanine ring and the amino hydrogen (-NH) at position 4 of cytosine. The nitrogen atom acts as a hydrogen bond acceptor, while the hydrogen of the cytosine amino group acts as a hydrogen bond donor. This third bond, often slightly weaker than the first two, still contributes significantly to the overall strength of the G-C base pair.
Energetic Considerations: Why Three is Better Than Two
The presence of three hydrogen bonds between guanine and cytosine contributes to a higher binding energy compared to the two hydrogen bonds in A-T base pairs. This increased stability is crucial for several reasons:
-
Higher Melting Temperature: DNA containing a higher proportion of G-C base pairs has a higher melting temperature (Tm). This means that more heat energy is required to denature or separate the two strands of the DNA molecule. This is a direct consequence of the increased hydrogen bonding strength.
-
Enhanced Stability in Diverse Environments: The stronger G-C bond enhances the stability of the DNA double helix in various environmental conditions, including variations in temperature, pH, and ionic strength. This robustness is essential for the integrity of genetic information.
-
Influence on DNA Structure: The stronger G-C bond subtly influences the overall structure and conformation of the DNA double helix. Regions rich in G-C base pairs tend to be more rigid and less prone to bending or deformation.
The Biological Significance of G-C Base Pairing
The specific and strong interaction between guanine and cytosine through hydrogen bonding plays a vital role in numerous biological processes:
-
Genetic Information Storage and Transmission: Accurate base pairing is critical for the faithful replication and transmission of genetic information. The precise interaction between G and C ensures that errors during DNA replication are minimized, preserving the integrity of the genetic code.
-
Gene Regulation: The G-C content of DNA sequences can influence gene expression and regulation. Promoter regions, for example, may have a specific G-C content that affects the binding of transcription factors.
-
DNA Structure and Stability: The G-C content of DNA plays a role in determining the overall structure and stability of the double helix. Regions with high G-C content are often more resistant to denaturation.
-
RNA Structure and Function: In RNA, G-C base pairing is essential for the formation of secondary and tertiary structures, including hairpin loops, stem-loops, and pseudoknots. These structures are crucial for the function of many RNA molecules, including tRNA, rRNA, and mRNA.
Beyond the Basics: Factors Influencing G-C Base Pairing
While the three hydrogen bonds are the primary force driving G-C base pairing, other factors also contribute:
-
Base Stacking: In addition to hydrogen bonding, base stacking interactions, involving hydrophobic interactions between the planar aromatic rings of the bases, contribute to the overall stability of the DNA double helix. These interactions are particularly significant in regions with high G-C content.
-
Solvent Effects: The surrounding water molecules play a role in influencing the hydrogen bonding interactions. The hydrogen bonding network between the bases and the water molecules affects the stability of the base pairs.
-
Ionic Strength: The concentration of ions in the solution can affect the electrostatic interactions between the charged groups on the bases, influencing the stability of the G-C base pairs.
Conclusion: A Foundation of Life
The hydrogen bonding interaction between guanine and cytosine is far more than just a simple chemical reaction. It represents a fundamental principle that underpins the very foundation of life. The specificity, strength, and consequences of this base pairing are integral to the stability, function, and evolution of all life forms. The intricate interplay of hydrogen bonding, base stacking, and solvent effects ensures the accurate replication and expression of genetic information, making G-C base pairing a truly remarkable phenomenon in the realm of molecular biology. Further research into the nuances of this interaction continues to reveal its profound impact on biological processes and expands our understanding of the remarkable complexity of life itself.
Latest Posts
Latest Posts
-
08 As A Fraction In Simplest Form
Mar 25, 2025
-
Which Of The Following Organisms Are Capable Of Photosynthesis
Mar 25, 2025
-
Water Is Pumped Steadily Out Of A Flooded Basement
Mar 25, 2025
-
What Is The Most Abundant Fossil Fuel
Mar 25, 2025
-
In A Eukaryotic Cell Dna Is Found In
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Guanine Forms Hydrogen Bonds With Cytosine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
