Write Iupac Name Of The Following Compound
News Leon
Apr 06, 2025 · 6 min read
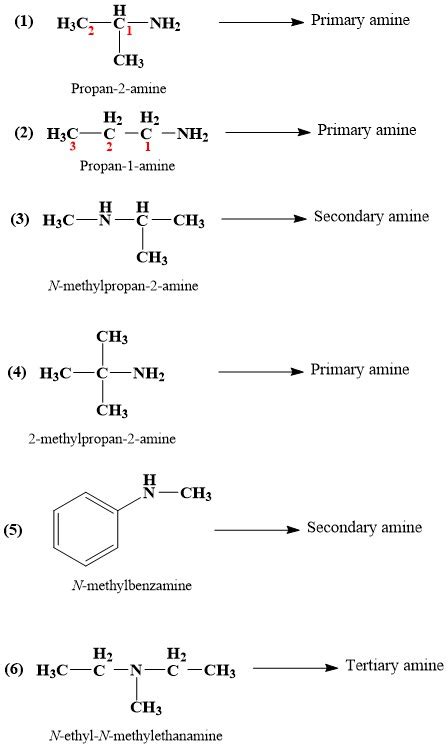
Table of Contents
Demystifying IUPAC Nomenclature: A Comprehensive Guide to Naming Organic Compounds
The International Union of Pure and Applied Chemistry (IUPAC) nomenclature is the globally accepted standardized system for naming chemical compounds. Understanding and applying IUPAC rules is crucial for chemists, students, and anyone working with chemical compounds. This comprehensive guide will delve into the intricacies of IUPAC nomenclature, focusing on strategies for accurately naming organic compounds, and providing examples to solidify your understanding. We will explore various functional groups and their naming conventions, addressing common challenges and misconceptions.
Understanding the Fundamentals of IUPAC Nomenclature
Before diving into complex molecules, let's establish the foundational principles of IUPAC nomenclature. The system is built upon a hierarchical structure, prioritizing the identification of the parent chain or parent structure. This is the longest continuous carbon chain in the molecule. Once the parent chain is identified, the following steps are crucial:
-
Identifying the Principal Functional Group: This is the functional group with the highest priority, determining the suffix of the name. The order of priority is crucial and follows a well-defined hierarchy. For example, carboxylic acids take precedence over alcohols, which take precedence over amines, and so on.
-
Numbering the Carbon Chain: The carbon chain is numbered to give the principal functional group the lowest possible number. This is essential for determining the position of substituents.
-
Identifying and Naming Substituents: These are atoms or groups of atoms attached to the parent chain. Each substituent is named and its position on the chain is indicated by a number.
-
Combining the Information: Finally, the information is combined to form the IUPAC name. This typically follows the format: [Prefix indicating substituents]-[Parent chain name]-[Suffix indicating principal functional group].
Prioritizing Functional Groups: A Hierarchy of Importance
The correct naming of a compound hinges on correctly identifying and prioritizing the functional groups present. The following list provides a general order of precedence (note that this is not exhaustive, and more complex scenarios may require deeper understanding of IUPAC rules):
-
Carboxylic acids (-COOH): The highest priority functional group, indicated by the suffix "-oic acid".
-
Anhydrides: Formed from the dehydration of two carboxylic acids.
-
Esters (-COO-): Characterized by the presence of a carbonyl group bonded to an oxygen atom.
-
Amides (-CONH₂): Contain a carbonyl group bonded to a nitrogen atom.
-
Nitriles (-CN): Containing a cyano group.
-
Aldehydes (-CHO): Possessing a carbonyl group at the end of a carbon chain.
-
Ketones (>C=O): Featuring a carbonyl group within the carbon chain.
-
Alcohols (-OH): Containing a hydroxyl group.
-
Amines (-NH₂): Characterized by an amino group.
-
Alkynes (-C≡C-): Containing a triple bond between two carbon atoms.
-
Alkenes (-C=C-): Having a double bond between two carbon atoms.
-
Alkanes (-C-C-): Saturated hydrocarbons with only single bonds.
Illustrative Examples: From Simple to Complex Structures
Let's work through several examples to solidify our understanding. Each example will break down the step-by-step process of applying IUPAC rules.
Example 1: A Simple Alkane
Consider the compound CH₃CH₂CH₂CH₃.
- Parent chain: Butane (four carbon atoms).
- Substituents: None.
- Principal functional group: Alkane (no higher priority group).
- IUPAC Name: Butane
Example 2: A Simple Alcohol
Consider the compound CH₃CH₂CH₂OH.
- Parent chain: Propane (three carbon atoms).
- Substituents: None.
- Principal functional group: Alcohol (-OH).
- Numbering: The hydroxyl group is on carbon 1.
- IUPAC Name: Propan-1-ol
Example 3: Branched Alkane with Substituents
Consider the compound CH₃CH(CH₃)CH₂CH₃.
- Parent chain: Butane (four carbon atoms).
- Substituents: One methyl group (-CH₃) on carbon 2.
- Principal functional group: Alkane.
- Numbering: The methyl group is assigned the lowest possible number.
- IUPAC Name: 2-Methylbutane
Example 4: Compound with Multiple Substituents
Consider the compound CH₃CH(CH₃)CH(CH₂CH₃)CH₃.
- Parent chain: Pentane (five carbon atoms).
- Substituents: One methyl group (-CH₃) on carbon 2 and one ethyl group (-CH₂CH₃) on carbon 3.
- Principal functional group: Alkane.
- Numbering: Substituents are given the lowest possible numbers. The alphabetization of substituents (ethyl before methyl) is applied.
- IUPAC Name: 3-Ethyl-2-methylpentane
Example 5: A Compound with a Ketone Functional Group
Consider the compound CH₃COCH₂CH₃.
- Parent chain: Butane (four carbon atoms).
- Substituents: None.
- Principal functional group: Ketone (>C=O).
- Numbering: The carbonyl group is on carbon 2.
- IUPAC Name: Butan-2-one
Example 6: A Compound with a Carboxylic Acid Functional Group
Consider the compound CH₃CH₂CH₂COOH.
- Parent chain: Butane (four carbon atoms).
- Substituents: None.
- Principal functional group: Carboxylic acid (-COOH). This takes precedence over any other functional group.
- Numbering: The carboxylic acid carbon is always carbon 1.
- IUPAC Name: Butanoic acid
Example 7: A More Complex Example with Multiple Functional Groups
Consider the compound CH₃CH(OH)CH₂COOH.
- Parent chain: Propane (three carbon atoms). The carboxylic acid group determines the parent chain.
- Substituents: One hydroxyl group (-OH) on carbon 2.
- Principal functional group: Carboxylic acid. The -OH group is a substituent, not the primary functional group.
- Numbering: The carboxylic acid carbon is carbon 1.
- IUPAC Name: 2-Hydroxypropanoic acid (the hydroxyl group is a substituent; the '-hydroxy' prefix is used).
Dealing with Complexities and Special Cases
IUPAC nomenclature can become considerably more complex when dealing with:
-
Cyclic Compounds: Naming cyclic compounds involves specifying the size of the ring, the presence and position of substituents, and the type of ring (e.g., cyclohexane, benzene).
-
Stereoisomers: Stereochemistry (cis/trans, E/Z, R/S) needs to be incorporated into the name to fully describe the molecule's three-dimensional structure.
-
Polyfunctional Compounds: When multiple functional groups of differing priorities are present, the highest-priority group determines the suffix, and lower-priority groups are represented as prefixes.
Conclusion: Mastering IUPAC Nomenclature
Mastering IUPAC nomenclature requires practice and a systematic approach. By following the established rules and prioritizing functional groups correctly, you can accurately name even the most complex organic compounds. Remember to consult the comprehensive IUPAC guidelines for more detailed and exhaustive rules. This guide provides a strong foundation for understanding the fundamental principles, enabling you to confidently name a wide range of organic molecules. Regular practice with diverse examples is key to developing proficiency in this crucial aspect of organic chemistry. Consistent effort and attention to detail are essential for success in this area. Remember, precise naming is fundamental to clear communication and collaboration within the scientific community.
Latest Posts
Latest Posts
-
Which Of The Following Statements About Leaders Is True
Apr 07, 2025
-
Find The Perimeter Of The Following Figure
Apr 07, 2025
-
Specific Heat Capacity Of Hydrogen Gas
Apr 07, 2025
-
Patents Copyrights And Trademarks Are Examples Of
Apr 07, 2025
-
Area Of Circle With Radius Of 7
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Write Iupac Name Of The Following Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
