Why Might Two Elements Possess Similar Chemical Properties
News Leon
Mar 18, 2025 · 6 min read
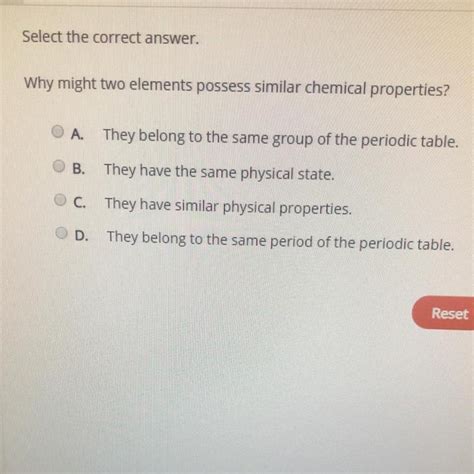
Table of Contents
Why Might Two Elements Possess Similar Chemical Properties?
Understanding why two elements might exhibit similar chemical properties is fundamental to grasping the organization and behavior of the periodic table. The answer lies primarily in their electronic configuration, specifically the arrangement of electrons in their outermost shell, known as the valence electrons. This article delves deep into this concept, exploring the various factors that contribute to chemical similarity, including the periodic trends and exceptions that occasionally arise.
The Role of Valence Electrons
The driving force behind similar chemical properties is the presence of a similar number and arrangement of valence electrons. These electrons are the outermost electrons in an atom, and they are the ones involved in chemical bonding. Atoms tend to react in ways that achieve a stable electron configuration, often resembling that of a noble gas (Group 18 elements) with a full outermost shell. This drive towards stability is the cornerstone of chemical reactivity.
Octet Rule and Stability
The octet rule, though not universally applicable, is a crucial guideline. It states that atoms tend to gain, lose, or share electrons to achieve eight electrons in their valence shell. This stable configuration minimizes energy, leading to greater stability. Elements with similar numbers of valence electrons will tend to participate in similar types of chemical reactions to achieve this stable octet. For example, elements in Group 1 (alkali metals) all have one valence electron, leading to a strong tendency to lose that electron and form +1 ions. Similarly, Group 17 (halogens) have seven valence electrons and readily gain one electron to form -1 ions.
Isoelectronic Species
Another important concept is that of isoelectronic species. These are atoms, ions, or molecules that have the same number of electrons. Although they might have different numbers of protons and neutrons, their identical electron configurations lead to surprisingly similar chemical behavior in certain contexts. For instance, the fluoride ion (F⁻) and the neon atom (Ne) are isoelectronic, both possessing 10 electrons. This shared electronic structure contributes to their similar chemical inertness.
Periodic Trends and Chemical Properties
The periodic table is arranged in a way that reflects the recurring patterns in electronic configuration. This organization directly impacts the chemical properties of elements.
Group Trends: The Power of Valence Electrons
Elements within the same group (vertical column) of the periodic table have the same number of valence electrons. This shared characteristic is the primary reason for their similar chemical properties. For example, the alkali metals (Group 1) are all highly reactive metals because they readily lose their single valence electron to form +1 ions. Similarly, the halogens (Group 17) are highly reactive nonmetals because they readily gain one electron to form -1 ions. This consistent behavior within a group is a direct consequence of their shared valence electron configuration.
Period Trends: Influence of Effective Nuclear Charge
Moving across a period (horizontal row), the number of valence electrons increases, leading to a gradual change in chemical properties. While elements in the same period don't exhibit the same degree of similarity as those in the same group, trends are still observable. For example, the electronegativity (the tendency to attract electrons in a chemical bond) generally increases across a period, as the effective nuclear charge (the positive charge experienced by valence electrons) increases. This increasing attraction for electrons affects the bonding characteristics and reactivity of elements within a period.
Exceptions and Nuances
While the valence electron configuration is the primary determinant of chemical properties, exceptions and nuances exist.
Transition Metals and Variable Oxidation States
Transition metals, located in the d-block of the periodic table, display a wider range of oxidation states (the charge of an ion) than main group elements. This stems from their ability to lose electrons from both their s and d orbitals. The multiple oxidation states lead to a greater diversity in chemical behavior, making it challenging to predict their properties solely based on their group position. For example, iron (Fe) can exist in +2 and +3 oxidation states, leading to significantly different chemical properties for each state.
Lanthanides and Actinides: Complex Behavior
The lanthanides and actinides (f-block elements) also present complexities. Their similar electronic configurations, with the gradual filling of the f-orbital, lead to remarkably similar chemical properties, often making separation and identification a significant challenge. This similarity, however, makes it difficult to establish strict periodic trends based solely on group position.
Influence of Atomic Size and Ionization Energy
Beyond valence electrons, other atomic properties also influence chemical behavior. Atomic size affects the ease with which an element can lose or gain electrons, influencing reactivity. Ionization energy, the energy required to remove an electron, is another crucial factor. Elements with low ionization energies tend to be more reactive metals, while those with high ionization energies tend to be less reactive nonmetals.
Beyond Simple Comparisons: Subtle Differences
Even within the same group, subtle differences in chemical properties can arise. This is because while valence electron configuration is paramount, other factors like atomic size, electronegativity, and the presence of d or f electrons can subtly modify reactivity. For instance, while all alkali metals readily lose one electron, their reactivity increases down the group due to increasing atomic size and decreasing ionization energy.
Applications and Importance
Understanding the relationship between electronic configuration and chemical properties has numerous practical applications.
Predicting Reactivity: Crucial for Chemical Synthesis
Predicting the reactivity of elements is essential for chemical synthesis and industrial processes. Knowing that elements in the same group share similar chemical properties allows chemists to anticipate reaction pathways and optimize synthesis strategies.
Material Science: Designing New Materials
In materials science, understanding periodic trends is crucial for designing new materials with desired properties. By carefully selecting elements with specific electronic configurations, scientists can tailor the properties of materials for various applications, ranging from electronics to construction.
Environmental Chemistry: Understanding Pollutant Behavior
In environmental chemistry, understanding the chemical properties of elements is vital for assessing the environmental impact of pollutants. Knowing how elements behave in different environments (e.g., water, soil, air) helps predict their toxicity and mobility.
Conclusion
The similarity in chemical properties between two elements primarily stems from the similarity in their valence electron configurations. Elements within the same group of the periodic table, possessing identical numbers of valence electrons, exhibit strikingly similar chemical behaviors. However, nuances and exceptions exist, primarily due to factors like atomic size, ionization energy, and the involvement of d and f orbitals. This comprehensive understanding of the relationship between electronic structure and chemical behavior forms the foundation for advanced chemical principles and many real-world applications across diverse fields. Further exploration into specific groups and periods and the study of subtle variations amongst elements will lead to a more nuanced and profound comprehension of the chemical world.
Latest Posts
Latest Posts
-
What Does 1 1 Ratio Mean
Mar 18, 2025
-
What Is 64 As A Fraction
Mar 18, 2025
-
The Way Of The World Analysis
Mar 18, 2025
-
Which Layer Of The Earth Is The Thinnest
Mar 18, 2025
-
What Part Of The Scapula Articulates With The Clavicle
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Why Might Two Elements Possess Similar Chemical Properties . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
