Why Can't The Subscripts Be Changed In A Chemical Equation
News Leon
Apr 06, 2025 · 6 min read
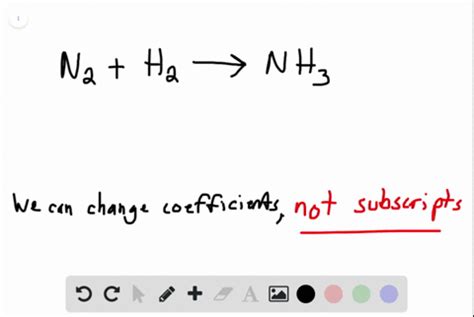
Table of Contents
Why Can't the Subscripts Be Changed in a Chemical Equation? The Importance of Maintaining Stoichiometric Integrity
Chemical equations are the fundamental language of chemistry, concisely representing the transformations of matter during chemical reactions. These equations adhere to strict rules, and one of the most crucial, yet often misunderstood, is the immutability of subscripts. Changing subscripts in a chemical equation fundamentally alters the identity of the substances involved, leading to inaccurate and misleading representations of the chemical process. This article delves into the reasons behind this inviolable rule, exploring the consequences of subscript alteration and highlighting the importance of maintaining stoichiometric integrity.
The Fundamental Role of Subscripts in Chemical Formulas
Subscripts in chemical formulas denote the number of atoms of each element present in a molecule or compound. For example, H₂O (water) indicates that each water molecule contains two hydrogen atoms and one oxygen atom. These subscripts are not arbitrary; they reflect the precise atomic ratios dictated by the chemical bonding within the molecule. These ratios are crucial for determining the molecule's properties, reactivity, and behavior in chemical reactions.
Distinguishing Between Subscripts and Coefficients
It's crucial to distinguish between subscripts and coefficients in chemical equations. Subscripts are part of the chemical formula and define the composition of a molecule. Coefficients, on the other hand, are placed before the chemical formula and indicate the number of molecules or moles of that substance involved in the reaction. Coefficients can be changed to balance a chemical equation, ensuring that the number of atoms of each element is the same on both the reactant and product sides. However, changing subscripts is strictly forbidden because it changes the fundamental nature of the chemical species.
The Consequences of Altering Subscripts
Altering subscripts in a chemical equation has drastic consequences, effectively creating a completely different chemical scenario. Let's illustrate this with an example:
Consider the balanced combustion reaction of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
If we were to incorrectly change the subscript of methane from CH₄ to CH₂, we would be representing a different chemical compound entirely – methene, a highly unstable and reactive molecule that behaves fundamentally differently from methane. This alteration would completely invalidate the equation and the implied reaction mechanism.
Similarly, altering the subscript of oxygen (O₂) to O would mean we are considering individual oxygen atoms instead of diatomic oxygen molecules. This has significant implications for reaction pathways and energy considerations, completely changing the nature of the combustion process. The resulting equation would no longer represent the actual chemical reaction, leading to erroneous predictions and potentially dangerous outcomes.
Impact on Molecular Structure and Properties
Subscripts directly reflect the molecular structure and, consequently, the physical and chemical properties of a substance. Changing a subscript fundamentally alters the molecular structure, leading to a compound with entirely different characteristics. For example, changing the subscript in H₂O to H₂O₂ transforms water into hydrogen peroxide, a powerful oxidizing agent with vastly different properties, including its reactivity, toxicity, and boiling point.
This difference in properties extends to all aspects of the chemical behavior of the substances. The reaction kinetics, thermodynamics, and even the physical state (solid, liquid, or gas) can significantly differ when subscripts are altered, rendering the equation completely irrelevant to the actual chemical process.
Maintaining Stoichiometric Integrity: The Law of Conservation of Mass
The inability to change subscripts is directly linked to the Law of Conservation of Mass, a cornerstone principle in chemistry. This law states that matter cannot be created or destroyed in a chemical reaction; only rearranged. A balanced chemical equation must adhere to this law, ensuring that the number of atoms of each element is the same on both the reactant and product sides.
Changing a subscript violates the law of conservation of mass because it alters the number of atoms of certain elements without accounting for their source or destination. For instance, changing H₂O to H₂O₂ implies the addition of an oxygen atom without specifying its origin, creating matter out of nothing, which is physically impossible.
Balancing Equations: The Role of Coefficients
Balancing a chemical equation involves adjusting the coefficients to ensure that the number of atoms of each element is the same on both sides. This process maintains the law of conservation of mass and accurately represents the chemical reaction without altering the inherent composition of the molecules involved. Coefficients reflect the relative amounts of reactants and products involved in the reaction, allowing for precise stoichiometric calculations and predictions.
Beyond Balancing: Subscripts and Chemical Understanding
Understanding the significance of subscripts extends beyond merely balancing equations. They provide crucial information about:
-
Molecular weight: Subscripts are essential for calculating the molecular weight or molar mass of a compound, a crucial parameter in various chemical calculations.
-
Empirical and molecular formulas: Subscripts are integral to determining the empirical and molecular formulas of compounds, allowing for precise identification and characterization of substances.
-
Reaction mechanisms: The precise composition of molecules, as defined by subscripts, is critical for understanding reaction mechanisms, explaining how reactants transform into products.
-
Chemical nomenclature: The system of chemical nomenclature relies on subscripts to communicate the precise composition of chemical compounds unambiguously.
The Practical Implications of Misinterpreting Subscripts
Misinterpreting or altering subscripts can have serious consequences in various contexts:
-
Laboratory experiments: Incorrectly interpreting chemical formulas can lead to inaccurate measurements, improper reactant ratios, and unsafe laboratory procedures.
-
Industrial processes: Errors in chemical equations can result in inefficient processes, wasted resources, and potentially hazardous outcomes in chemical manufacturing.
-
Environmental science: Accurate chemical equations are critical for modeling and understanding environmental processes, such as pollution control and remediation.
-
Medical applications: Accurate chemical equations are crucial in drug development, pharmacology, and medical diagnostics. An error in the chemical formula of a drug, even a subtle change in a subscript, could have devastating health consequences.
Conclusion: The Inviolable Rule of Subscripts
Changing subscripts in a chemical equation is not just a matter of mathematical precision; it's a fundamental error that undermines the very principles of chemistry. It invalidates the equation, misrepresents the chemical process, and can lead to dangerous and inaccurate conclusions. The immutability of subscripts is not merely a rule to be followed blindly; it is a direct consequence of the fundamental laws of chemistry, particularly the law of conservation of mass, and its understanding is essential for a thorough grasp of chemical principles and their practical applications. By respecting and understanding this inviolable rule, we ensure accurate chemical representations and predictions, paving the way for safe and effective advancements in various scientific and technological fields. The correct use and interpretation of subscripts are critical for accurate chemical representation and prediction. Mastering this aspect of chemical notation is fundamental to success in chemistry and related disciplines.
Latest Posts
Latest Posts
-
Area Of Circle With Radius 10
Apr 09, 2025
-
Provides Mechanical Supports And Anchorage To The Cell
Apr 09, 2025
-
Which Solution Has The Highest Boiling Point At Standard Pressure
Apr 09, 2025
-
How Many Neutrons Does Xe Have
Apr 09, 2025
-
Do Homologous Chromosomes Pair In Mitosis
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Why Can't The Subscripts Be Changed In A Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.