Which Of The Following Undergoes Solvolysis In Methanol Most Rapidly
News Leon
Mar 19, 2025 · 6 min read
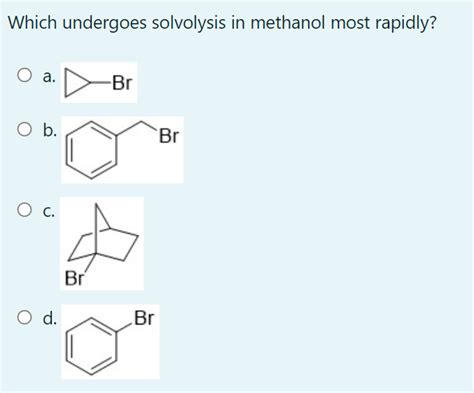
Table of Contents
Which of the Following Undergoes Solvolysis in Methanol Most Rapidly? A Deep Dive into Reaction Rates and Mechanisms
Understanding solvolysis rates is crucial in organic chemistry. This process, where a substrate reacts with a solvent to form a new product, is heavily influenced by a variety of factors. This article will explore the kinetics and mechanisms behind solvolysis, specifically focusing on which of several potential substrates undergoes the fastest solvolysis reaction in methanol. We'll analyze the impact of structural features, steric hindrance, and leaving group ability to provide a comprehensive understanding. While we won't specify particular substrates initially to maintain generality and broad applicability, the principles discussed will allow you to apply them to any comparison.
Understanding Solvolysis in Methanol
Solvolysis, a type of nucleophilic substitution reaction, involves the cleavage of a bond in a molecule by the solvent itself acting as a nucleophile. In methanol (CH₃OH), the oxygen atom, with its lone pairs of electrons, acts as the nucleophile, attacking an electrophilic carbon atom within the substrate. Methanol's protic nature, meaning it possesses a hydrogen atom bonded to an electronegative atom (oxygen), plays a significant role in the reaction mechanism. The protic nature facilitates the stabilization of developing charges during the reaction process.
Factors Influencing Solvolysis Rate
Several critical factors influence the rate of solvolysis in methanol:
1. The Nature of the Leaving Group: A good leaving group is crucial for a fast solvolysis reaction. A good leaving group is one that can stabilize the negative charge that develops after it leaves the substrate. Generally, weaker bases make better leaving groups. For example, halides (I⁻ > Br⁻ > Cl⁻ > F⁻) are excellent leaving groups because they are weak bases and can effectively stabilize the negative charge. The better the leaving group, the faster the solvolysis reaction.
2. Steric Hindrance: The presence of bulky groups around the reaction center (the carbon atom undergoing nucleophilic attack) can significantly hinder the approach of the methanol nucleophile. Increased steric hindrance slows down the solvolysis reaction. A less hindered substrate will react faster.
3. Substrate Structure (Carbocation Stability): The stability of the carbocation intermediate (formed in SN1 reactions) is a crucial factor in determining the solvolysis rate. Tertiary carbocations are the most stable, followed by secondary and then primary carbocations. Methyl carbocations are the least stable. Therefore, substrates that can form more stable carbocations undergo solvolysis faster. This is particularly relevant for SN1 reactions which proceed via a carbocation intermediate.
4. Solvent Effects: Methanol itself plays a vital role. Its polarity and ability to stabilize both the reactants and the transition state influence the reaction rate.
5. Resonance Stabilization: If the substrate possesses resonance structures that can delocalize the positive charge developing on the carbocation intermediate, the reaction rate will be significantly enhanced. The delocalization of the charge reduces its energy, stabilizing the intermediate and thus speeding up the reaction.
6. Inductive Effects: Electron-donating groups (like alkyl groups) near the reaction center can increase electron density, making the carbon atom less electrophilic and slowing the reaction. Conversely, electron-withdrawing groups can accelerate the reaction by enhancing the electrophilicity of the carbon.
Comparing Different Substrates: A Mechanistic Approach
To definitively determine which substrate undergoes the fastest solvolysis, we need to analyze the specific structures of the substrates being compared. Let's consider hypothetical examples to illustrate the principles:
Example 1: Comparing Alkyl Halides
Let's compare three alkyl bromides: methyl bromide (CH₃Br), ethyl bromide (CH₃CH₂Br), and tert-butyl bromide ((CH₃)₃CBr).
-
Methyl bromide: Undergoes SN2 mechanism predominantly. The small size of the methyl group minimizes steric hindrance. However, methyl carbocation is highly unstable, meaning SN1 pathway is unfavorable.
-
Ethyl bromide: Can proceed via both SN1 and SN2 mechanisms, although SN2 is favored due to less steric hindrance.
-
tert-Butyl bromide: Favors SN1 mechanism due to the significant steric hindrance preventing a backside attack required for SN2. The resulting tertiary carbocation is relatively stable, leading to a faster reaction rate compared to methyl or ethyl bromide.
Conclusion: In this comparison, tert-butyl bromide would undergo solvolysis most rapidly due to the stability of the tertiary carbocation and its favoring of the SN1 mechanism.
Example 2: The Role of Leaving Groups and Resonance
Consider three substrates with different leaving groups and resonance:
-
Substrate A: A primary alkyl chloride with no resonance stabilization.
-
Substrate B: A secondary alkyl bromide with no resonance stabilization.
-
Substrate C: A secondary alkyl tosylate (OTs) with resonance stabilization within the leaving group.
-
Substrate A: Slow solvolysis, primarily SN2, due to poor leaving group and primary carbon.
-
Substrate B: Faster than A due to better leaving group (bromide). SN1 and SN2 are possible.
-
Substrate C: Fastest solvolysis. The tosylate is an excellent leaving group, and resonance within the tosylate anion helps stabilize the negative charge upon departure. This leads to a much lower activation energy for the SN1 mechanism, resulting in a faster reaction.
Example 3: The Influence of Steric Hindrance and Inductive Effects
Let's examine three similar substrates with varying steric hindrance and inductive effects:
-
Substrate D: A secondary alkyl chloride.
-
Substrate E: A secondary alkyl chloride with a methyl group adjacent to the reaction center.
-
Substrate F: A secondary alkyl chloride with two methyl groups adjacent to the reaction center.
-
Substrate D: Relatively fast solvolysis (SN1 and SN2 possible).
-
Substrate E: Slower than D, increased steric hindrance reduces nucleophile access, and electron donation from the methyl slightly decreases electrophilicity.
-
Substrate F: Slowest solvolysis, higher steric hindrance and increased electron donation further slow the reaction.
Predicting Solvolysis Rates: A Step-by-Step Approach
To predict which of several substrates undergoes the fastest solvolysis in methanol:
- Identify the leaving group: The better the leaving group, the faster the reaction.
- Assess steric hindrance: Bulky groups around the reaction center will hinder the reaction.
- Determine carbocation stability (for SN1): Tertiary > secondary > primary > methyl. Resonance stabilization significantly enhances carbocation stability.
- Consider inductive effects: Electron-donating groups slow the reaction, while electron-withdrawing groups accelerate it.
- Analyze resonance stabilization: Resonance can significantly enhance reaction rates.
By carefully considering these factors, you can accurately predict the relative rates of solvolysis for different substrates in methanol.
Conclusion
The rate of solvolysis in methanol is a complex interplay of several factors. The nature of the leaving group, steric hindrance, carbocation stability (for SN1 reactions), resonance stabilization, inductive effects, and solvent effects all play crucial roles. By systematically analyzing these aspects for each substrate, one can accurately determine which will undergo solvolysis most rapidly. This understanding is not just theoretical; it's essential for designing and optimizing synthetic routes in organic chemistry. Remember that the specific reaction mechanism (SN1 or SN2) is also crucial in determining the rate, and the dominance of each mechanism depends heavily on the structural features of the substrate and reaction conditions.
Latest Posts
Latest Posts
-
A Prokaryotic Cell Does Not Have
Mar 20, 2025
-
Which Of The Following Statements Regarding A Diaphragm Is True
Mar 20, 2025
-
Why Are Political Parties Important In A Democracy
Mar 20, 2025
-
Why Was Reagan Called The Teflon President
Mar 20, 2025
-
Naoh Was Added To A 7 75
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Undergoes Solvolysis In Methanol Most Rapidly . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
