Which Of The Following Is Weak Electrolyte
News Leon
Mar 27, 2025 · 6 min read
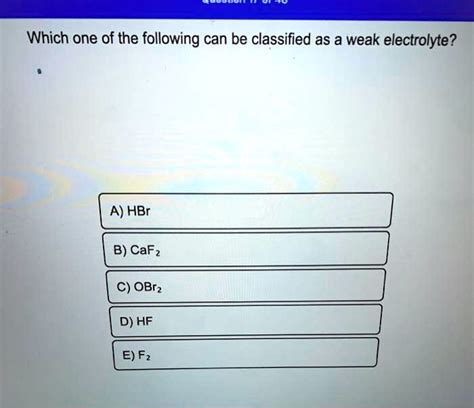
Table of Contents
Which of the following is a weak electrolyte? Understanding Electrolyte Strength
Determining whether a substance is a strong or weak electrolyte is crucial in chemistry, impacting various applications from understanding solution conductivity to predicting reaction rates. This article delves deep into the concept of weak electrolytes, contrasting them with strong electrolytes, and providing a comprehensive guide to identifying them. We'll examine various factors that influence electrolyte strength and provide examples to solidify your understanding.
What are Electrolytes?
Before diving into the specifics of weak electrolytes, let's establish a foundational understanding of electrolytes themselves. Electrolytes are substances that, when dissolved in a polar solvent like water, produce a solution that conducts electricity. This conductivity arises from the presence of mobile charged particles, namely ions, within the solution. The process of producing these ions is called ionization or dissociation.
The ability of a substance to conduct electricity depends directly on the concentration of these mobile ions. The higher the concentration of ions, the greater the conductivity. This is why strong electrolytes are significant conductors, while weak electrolytes exhibit much lower conductivity.
Strong Electrolytes vs. Weak Electrolytes: A Key Distinction
The crucial difference lies in the extent to which a substance dissociates into ions when dissolved.
-
Strong electrolytes completely dissociate into ions in solution. This means that virtually every molecule of the strong electrolyte breaks apart into its constituent ions. Examples include strong acids (like HCl, HNO₃, H₂SO₄), strong bases (like NaOH, KOH, Ba(OH)₂), and many soluble salts (like NaCl, KCl, MgCl₂).
-
Weak electrolytes only partially dissociate into ions in solution. A significant portion of the weak electrolyte remains in its molecular form, undissociated. This leads to a lower concentration of ions compared to strong electrolytes, resulting in lower conductivity. Examples include weak acids (like acetic acid, CH₃COOH), weak bases (like ammonia, NH₃), and some sparingly soluble salts.
In essence: Strong electrolytes are almost 100% ionized in solution, while weak electrolytes are only partially ionized (typically less than 10% ionized).
Identifying Weak Electrolytes: Key Characteristics and Examples
Several factors contribute to a substance's classification as a weak electrolyte. These factors help us predict and understand their behavior in solution:
1. Weak Acids and Weak Bases
The most common examples of weak electrolytes are weak acids and weak bases. These substances only partially donate or accept protons (H⁺ ions) in aqueous solution.
-
Weak Acids: These acids do not fully dissociate into their conjugate base and H⁺ ions. Instead, an equilibrium is established between the undissociated acid and its ions. The extent of dissociation is expressed by the acid dissociation constant, Ka. A smaller Ka value indicates a weaker acid and thus, a weaker electrolyte. Examples include:
- Acetic acid (CH₃COOH): Found in vinegar, it's a common weak acid used in various applications.
- Formic acid (HCOOH): Present in ant stings, it's another example of a weak acid.
- Hydrocyanic acid (HCN): A highly toxic weak acid.
- Benzoic acid (C₇H₆O₂): A weak acid used as a preservative.
-
Weak Bases: Similar to weak acids, weak bases do not fully ionize in solution. They only partially accept protons, establishing an equilibrium between the undissociated base and its ions. The extent of dissociation is expressed by the base dissociation constant, Kb. A smaller Kb value indicates a weaker base and thus, a weaker electrolyte. Examples include:
- Ammonia (NH₃): Commonly used in cleaning products, it's a weak base.
- Methylamine (CH₃NH₂): An organic weak base.
- Pyridine (C₅H₅N): A heterocyclic aromatic weak base.
2. Sparingly Soluble Salts
Certain salts, despite being ionic compounds, exhibit low solubility in water. This means only a small amount dissolves, leading to a low concentration of ions and thus, weak electrolyte behavior. Solubility product constants (Ksp) are used to quantify the solubility of these salts. A small Ksp value implies low solubility and weak electrolyte behavior. Examples include:
- Silver chloride (AgCl): Used in photographic film, it's a sparingly soluble salt.
- Lead(II) sulfate (PbSO₄): A sparingly soluble salt, relevant in battery chemistry.
- Calcium carbonate (CaCO₃): A component of limestone and marble, exhibiting low solubility.
3. Factors Affecting Electrolyte Strength
Several factors can influence the strength of an electrolyte:
- Polarity of the solvent: Polar solvents, like water, effectively solvate ions, promoting dissociation. Non-polar solvents hinder ion dissociation.
- Temperature: Increasing temperature usually increases the extent of dissociation for weak electrolytes.
- Concentration: The concentration of the electrolyte in solution also affects the extent of dissociation. Diluting a weak electrolyte solution can slightly increase the percentage of dissociation. However, the overall concentration of ions might decrease.
- Nature of the solute: The inherent properties of the solute molecule, such as its bond strength and molecular structure, dictate its tendency to dissociate into ions.
Practical Applications and Significance
Understanding the difference between strong and weak electrolytes is vital in numerous chemical and practical applications:
-
Conductivity measurements: The conductivity of a solution is directly related to the concentration of ions. This allows us to determine the strength of an electrolyte based on conductivity measurements.
-
Electrochemical cells: In batteries and other electrochemical cells, the strength of the electrolytes used significantly impacts their performance and efficiency.
-
Chemical reactions: The extent of ionization affects reaction rates and equilibrium positions in many chemical reactions involving electrolytes.
-
Biological systems: Many biological processes rely on the presence and behavior of electrolytes, including the transmission of nerve impulses and muscle contraction. Maintaining proper electrolyte balance is crucial for health.
-
Water treatment: Understanding electrolyte behavior is important in various water treatment processes, such as water softening and purification.
Illustrative Examples and Practice Problems
Let's solidify your understanding with some examples and practice problems:
Example 1: Which of the following is a weak electrolyte: HCl, CH₃COOH, NaCl, NaOH?
Answer: CH₃COOH (acetic acid) is the weak electrolyte. HCl, NaCl, and NaOH are all strong electrolytes.
Example 2: Explain why a solution of ammonia (NH₃) is a weak conductor of electricity.
Answer: Ammonia is a weak base. When dissolved in water, it only partially reacts with water to form ammonium ions (NH₄⁺) and hydroxide ions (OH⁻). The relatively low concentration of these ions results in weak conductivity.
Example 3: Compare the conductivity of 0.1 M solutions of HCl and CH₃COOH. Explain the difference.
Answer: The 0.1 M HCl solution will have significantly higher conductivity compared to the 0.1 M CH₃COOH solution. HCl is a strong acid and completely dissociates into H⁺ and Cl⁻ ions, resulting in a high concentration of charge carriers. CH₃COOH, being a weak acid, only partially dissociates, resulting in a much lower concentration of ions and therefore lower conductivity.
Example 4: How does the temperature affect the conductivity of a weak electrolyte solution?
Answer: Increasing the temperature generally increases the conductivity of a weak electrolyte solution. Higher temperatures increase the kinetic energy of the molecules, leading to increased collisions and a higher percentage of dissociation into ions, thereby increasing the number of charge carriers.
By carefully considering the characteristics outlined above and practicing with these examples, you can confidently identify weak electrolytes and understand their importance in various chemical and practical contexts. Remember that the key difference between strong and weak electrolytes lies in the extent of their ionization or dissociation in solution. Weak electrolytes only partially dissociate, leading to lower conductivity and distinct chemical behavior compared to their strong counterparts.
Latest Posts
Latest Posts
-
Which Layer Does Weather Occur In
Mar 30, 2025
-
Economic Growth Is Depicted By
Mar 30, 2025
-
Which Of The Following Is A Conductor
Mar 30, 2025
-
Frame A Sentence Using The Word
Mar 30, 2025
-
The Broadest Taxonomic Group For Classifying Living Organisms Is The
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is Weak Electrolyte . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
