Which Of The Following Is Most Soluble In Water
News Leon
Mar 24, 2025 · 6 min read
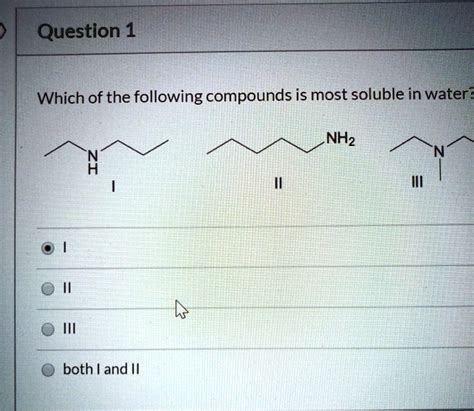
Table of Contents
Which of the Following is Most Soluble in Water? A Deep Dive into Solubility
Solubility, the ability of a substance to dissolve in a solvent, is a fundamental concept in chemistry with far-reaching implications across various fields, from medicine and environmental science to industrial processes and everyday life. Understanding solubility requires considering several factors, including the nature of the solute (the substance being dissolved), the nature of the solvent (the substance doing the dissolving), temperature, and pressure. This article will explore the principles governing solubility, focusing on how to determine which of several substances is most soluble in water, a ubiquitous and incredibly important solvent.
Understanding the Basics: "Like Dissolves Like"
The adage "like dissolves like" is a crucial starting point for predicting solubility. This principle highlights the importance of intermolecular forces. Substances with similar intermolecular forces tend to be more soluble in each other. Water, a polar molecule with strong hydrogen bonding, readily dissolves substances with polar characteristics or those capable of forming hydrogen bonds. Conversely, nonpolar substances, those with weak London dispersion forces, are generally insoluble in water.
Let's break down the common types of intermolecular forces:
-
Hydrogen Bonding: The strongest type of dipole-dipole interaction, occurring between molecules containing hydrogen atoms bonded to highly electronegative atoms like oxygen (O), nitrogen (N), or fluorine (F). Water itself is held together by extensive hydrogen bonding.
-
Dipole-Dipole Interactions: Occur between polar molecules, where one end of the molecule carries a partial positive charge (δ+) and the other a partial negative charge (δ-). These partial charges attract each other.
-
London Dispersion Forces (LDFs): These are the weakest intermolecular forces and are present in all molecules, regardless of polarity. They arise from temporary fluctuations in electron distribution, creating temporary dipoles. LDFs are stronger in larger molecules with more electrons.
-
Ion-Dipole Interactions: Occur between ions (charged particles) and polar molecules. The positive end of the polar molecule attracts anions (negatively charged ions), while the negative end attracts cations (positively charged ions).
Factors Affecting Solubility
Beyond the "like dissolves like" principle, several other factors influence solubility:
-
Temperature: The effect of temperature on solubility is often complex and depends on whether the dissolution process is exothermic (releases heat) or endothermic (absorbs heat). Generally, the solubility of most solids in liquids increases with increasing temperature, while the solubility of gases in liquids decreases with increasing temperature.
-
Pressure: Pressure significantly impacts the solubility of gases in liquids. According to Henry's Law, the solubility of a gas is directly proportional to the partial pressure of that gas above the liquid. Increased pressure leads to increased solubility for gases. Pressure has a negligible effect on the solubility of solids and liquids.
-
Molecular Structure: The shape and size of molecules play a role in solubility. Branched molecules often have lower solubility than their straight-chain isomers due to differences in packing efficiency. Larger molecules generally have lower solubility unless strong intermolecular forces compensate.
Comparing Solubility: A Case Study
Let's consider a hypothetical scenario: you're given a selection of substances and asked to determine which is most soluble in water. The substances are:
- Sodium Chloride (NaCl): An ionic compound.
- Octane (C₈H₁₈): A nonpolar hydrocarbon.
- Ethanol (C₂H₅OH): A polar molecule with hydrogen bonding capabilities.
- Benzene (C₆H₆): A nonpolar aromatic hydrocarbon.
- Glucose (C₆H₁₂O₆): A polar molecule with multiple hydroxyl (-OH) groups capable of hydrogen bonding.
Analysis:
-
Sodium Chloride (NaCl): This ionic compound readily dissolves in water due to strong ion-dipole interactions. Water molecules surround the Na⁺ and Cl⁻ ions, effectively separating them and dissolving the crystal lattice. High solubility.
-
Octane (C₈H₁₈): This nonpolar hydrocarbon has only weak London dispersion forces. There is minimal interaction with water molecules, resulting in very low solubility. Very low solubility.
-
Ethanol (C₂H₅OH): This polar molecule can form hydrogen bonds with water molecules due to its hydroxyl (-OH) group. The hydrogen bonding leads to significant solubility. High solubility.
-
Benzene (C₆H₆): This nonpolar aromatic hydrocarbon relies solely on weak London dispersion forces for interaction with water. Its insolubility is similar to that of octane. Very low solubility.
-
Glucose (C₆H₁₂O₆): Glucose has multiple hydroxyl (-OH) groups, allowing for extensive hydrogen bonding with water molecules. This leads to relatively high solubility. High solubility.
Conclusion: In this example, both Sodium Chloride and Ethanol exhibit high solubility in water, while Glucose also shows high solubility. The relative solubility between NaCl and Ethanol and Glucose would depend on factors like temperature and concentration, but all three substantially exceed the solubility of Octane and Benzene. The most soluble will likely be NaCl, but the difference isn't extreme. Glucose might be more soluble at lower temperatures as it is more likely to form a supersaturated solution, while NaCl is more soluble at higher temperatures, especially above 100°C.
Advanced Considerations: Solubility Product (Ksp) and Other Equilibrium Constants
For ionic compounds, the extent of solubility is quantified using the solubility product constant (Ksp). Ksp represents the equilibrium constant for the dissolution of an ionic compound in water. A higher Ksp indicates greater solubility.
For other substances, various equilibrium constants describe their solubility behavior depending on the nature of the dissolution process.
Practical Applications: Solubility in Everyday Life and Scientific Research
Understanding solubility is crucial in numerous practical applications:
-
Pharmaceutical Industry: Solubility dictates the bioavailability of drugs. Drugs must be soluble enough to be absorbed into the bloodstream. Formulations often involve strategies to enhance solubility.
-
Environmental Science: Solubility plays a critical role in determining the fate and transport of pollutants in the environment. Solubility affects the mobility and bioavailability of contaminants.
-
Industrial Processes: Many industrial processes rely on selective dissolution of substances. For example, separating valuable metals from ores often involves carefully controlling solubility.
-
Food Science: Solubility influences the texture and flavor of food products. The solubility of various ingredients affects their distribution and interaction in food matrices.
Conclusion: Solubility – A Multifaceted Concept
Determining which substance is most soluble in water requires careful consideration of intermolecular forces, temperature, pressure, and molecular structure. The "like dissolves like" principle serves as a valuable guideline. Quantitative measures like the solubility product constant (Ksp) provide a more precise assessment for ionic compounds. Understanding solubility is essential across numerous scientific disciplines and everyday applications. This article has provided a comprehensive overview of solubility principles and their relevance, equipping readers with the knowledge to analyze and predict solubility in various contexts. Remember to always consider the specific conditions (temperature, pressure) to provide the most accurate assessment.
Latest Posts
Latest Posts
-
Which Of The Following Are Characteristics Of Prokaryotic Cells
Mar 28, 2025
-
Which Is The Most Polar Bond
Mar 28, 2025
-
What Color Does Litmus Paper Turn In Water
Mar 28, 2025
-
Explain The Gradation In Reactivity Of Halogen Family
Mar 28, 2025
-
A Pectoral Girdle Consists Of Two Bones The And The
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is Most Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
