Which Of The Following Is A Correctly Balanced Equation
News Leon
Mar 20, 2025 · 5 min read
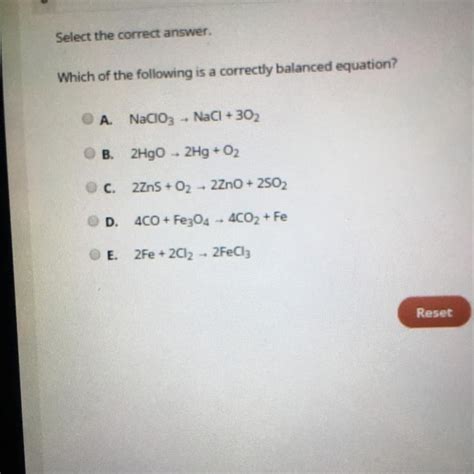
Table of Contents
Which of the Following is a Correctly Balanced Equation? A Deep Dive into Chemical Stoichiometry
Balancing chemical equations is a fundamental concept in chemistry. It's the cornerstone of understanding stoichiometry, allowing us to predict the quantities of reactants needed and products formed in a chemical reaction. A balanced equation adheres to the law of conservation of mass, stating that matter cannot be created or destroyed in a chemical reaction; only rearranged. This means the number of atoms of each element must be the same on both the reactant and product sides of the equation. This article will explore the principles of balancing equations, common pitfalls, and provide a step-by-step approach to determine which of a given set of equations is correctly balanced.
Understanding Chemical Equations
Before delving into balancing, let's refresh our understanding of chemical equations. A chemical equation is a symbolic representation of a chemical reaction, using chemical formulas to represent the reactants (starting materials) and products (resulting substances). For example:
Reactants → Products
The arrow (→) indicates the direction of the reaction. Reactants are written on the left side, and products are written on the right. For example, the combustion of methane (CH₄) can be represented as:
CH₄ + O₂ → CO₂ + H₂O
This equation, however, is unbalanced. Let's analyze why.
The Importance of Balancing Equations
An unbalanced equation doesn't accurately reflect the reality of a chemical reaction. It violates the law of conservation of mass. In the unbalanced methane combustion equation above, we have:
- Reactants: 1 Carbon atom, 4 Hydrogen atoms, 2 Oxygen atoms
- Products: 1 Carbon atom, 2 Hydrogen atoms, 3 Oxygen atoms
The number of oxygen and hydrogen atoms differs between the reactant and product sides. A balanced equation ensures that the number of atoms of each element remains consistent throughout the reaction. This allows us to perform stoichiometric calculations, determining the relative amounts of reactants and products involved. This is crucial in various applications, including:
- Industrial Chemistry: Optimizing reaction yields and minimizing waste.
- Pharmaceutical Industry: Precisely determining drug dosages and synthesis pathways.
- Environmental Science: Assessing the impact of chemical reactions on the environment.
Methods for Balancing Chemical Equations
Several methods can be used to balance chemical equations. The most common approaches include:
1. Inspection Method (Trial and Error)
This is the simplest method, involving systematically adjusting the coefficients (the numbers placed in front of chemical formulas) until the number of atoms of each element is equal on both sides. It often involves a bit of trial and error. Let's balance the methane combustion equation using this method:
Unbalanced: CH₄ + O₂ → CO₂ + H₂O
- Balance Carbon: Carbon is already balanced (1 on each side).
- Balance Hydrogen: There are 4 hydrogen atoms on the left and 2 on the right. We need to add a coefficient of 2 to H₂O:
CH₄ + O₂ → CO₂ + 2H₂O
- Balance Oxygen: Now we have 4 oxygen atoms on the right (2 in CO₂ and 2 in 2H₂O) and 2 on the left. We add a coefficient of 2 to O₂:
CH₄ + 2O₂ → CO₂ + 2H₂O
Now the equation is balanced. Both sides have 1 carbon atom, 4 hydrogen atoms, and 4 oxygen atoms.
2. Algebraic Method
This method uses algebra to solve for the coefficients. We assign variables to each coefficient and create a system of equations based on the number of atoms of each element. Let's use the same methane combustion example:
CH₄ + aO₂ → bCO₂ + cH₂O
Creating equations based on the number of atoms:
- Carbon: 1 = b
- Hydrogen: 4 = 2c
- Oxygen: 2a = 2b + c
Solving these equations gives us a = 2, b = 1, and c = 2. Substituting these values back into the original equation gives us the balanced equation:
CH₄ + 2O₂ → CO₂ + 2H₂O
3. Oxidation-Reduction (Redox) Method
This method is used for more complex reactions involving electron transfer. It involves identifying the oxidation and reduction half-reactions and balancing them separately before combining them. This method is beyond the scope of this introductory article but is essential for understanding complex redox reactions.
Common Pitfalls in Balancing Equations
Several common mistakes can lead to incorrectly balanced equations:
- Changing subscripts: Subscripts within a chemical formula indicate the number of atoms of each element within that molecule. Changing subscripts alters the chemical formula itself, creating a different substance entirely. Coefficients are the only numbers you should change when balancing equations.
- Ignoring polyatomic ions: In reactions involving polyatomic ions (like sulfate, SO₄²⁻), treat the ion as a single unit when balancing. Adjust the coefficients to balance the entire ion, rather than individual atoms within the ion.
- Not double-checking: Always verify the balanced equation by counting the atoms of each element on both sides to ensure they are equal.
Example: Determining Correctly Balanced Equations
Let's say you are given the following equations and asked to identify the correctly balanced equation:
Equation 1: 2H₂ + O₂ → 2H₂O
Equation 2: H₂ + O₂ → H₂O
Equation 3: H₂ + 2O₂ → 2H₂O
Equation 4: 2H₂ + 2O₂ → 2H₂O
Analysis:
-
Equation 1: This equation is correctly balanced. There are 4 hydrogen atoms and 2 oxygen atoms on both sides.
-
Equation 2: This equation is unbalanced. There are 2 oxygen atoms on the left and only 1 on the right.
-
Equation 3: This equation is unbalanced. There are 2 hydrogen atoms on the left and 4 oxygen atoms on the right; this violates the law of conservation of mass.
-
Equation 4: This equation is unbalanced. There are 4 hydrogen atoms and 4 oxygen atoms on the left, but only 2 oxygen atoms on the right.
Therefore, only Equation 1 is a correctly balanced equation.
Conclusion
Balancing chemical equations is a crucial skill in chemistry, essential for understanding stoichiometry and performing quantitative calculations. While the inspection method may suffice for simpler equations, the algebraic method offers a more systematic approach for more complex reactions. Remember always to adjust coefficients, not subscripts, and double-check your work to ensure the number of atoms of each element is equal on both sides of the equation. Mastering this skill allows for accurate predictions and calculations, crucial in various scientific and industrial applications. By understanding the principles and applying the appropriate methods, you can confidently determine which equation is correctly balanced, ensuring accuracy in your chemical calculations and interpretations. Consistent practice and attention to detail are key to mastering this fundamental aspect of chemistry.
Latest Posts
Latest Posts
-
The Size Of A Cell Is Limited By The
Mar 20, 2025
-
How Many Trips Around The Sun In A Year
Mar 20, 2025
-
What Is The Degree Of 9
Mar 20, 2025
-
Are Frogs Omnivores Herbivores Or Carnivores
Mar 20, 2025
-
How Many Years Is A Four Score
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Correctly Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
