Which Of The Following Ions Is Aromatic
News Leon
Mar 14, 2025 · 6 min read
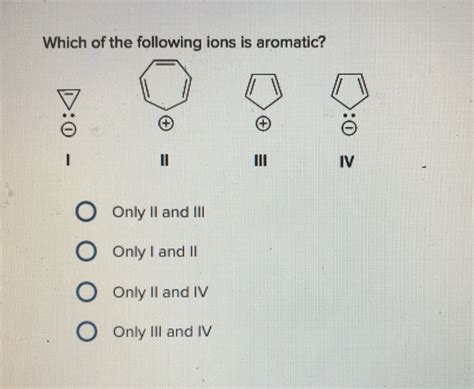
Table of Contents
Which of the Following Ions is Aromatic? A Deep Dive into Aromaticity
Determining aromaticity is a crucial skill in organic chemistry. Understanding the criteria for aromaticity allows us to predict the reactivity and stability of various cyclic compounds. This article delves into the intricacies of aromaticity, examining the rules and applying them to identify aromatic ions among a given set. We’ll explore the concept systematically, providing clear examples and explanations.
Understanding Aromaticity: The Huckel's Rule and Beyond
Aromatic compounds are characterized by exceptional stability, a property stemming from their unique electronic structure. This stability is explained primarily by Hückel's rule, a cornerstone of aromatic chemistry. Hückel's rule states that a planar, cyclic, conjugated system is aromatic if it contains (4n + 2) π electrons, where 'n' is a non-negative integer (n = 0, 1, 2, 3...).
However, Hückel's rule is not the sole criterion. Three crucial conditions must be met for a compound to be considered aromatic:
- Cyclic: The molecule must possess a closed ring structure.
- Planar: All atoms in the ring must lie in the same plane. This allows for effective overlap of p-orbitals.
- Conjugated: The molecule must have a continuous system of overlapping p-orbitals encompassing all atoms within the ring. This continuous overlap is crucial for delocalization of π electrons.
Let's break down each condition and explore its significance in determining aromaticity.
The Importance of Planarity: Why Flatness Matters
Planarity is essential because it enables the effective sideways overlap of p-orbitals, crucial for forming delocalized π molecular orbitals. If the ring is not planar, the p-orbital overlap is disrupted, hindering the formation of the delocalized π electron system. Steric hindrance, ring strain, or the presence of sp³ hybridized atoms can prevent a molecule from achieving planarity. For example, cyclooctatetraene, despite having 8 π electrons (which seems to fit 4n, anti-aromatic), is non-aromatic due to its non-planar structure. It adopts a tub-shaped conformation to minimize angle strain.
Conjugation: The Continuous Delocalization Network
Conjugation refers to the continuous alternating system of single and double bonds (or lone pairs) that allows for the delocalization of electrons. This delocalization stabilizes the molecule. The continuous overlap of p-orbitals above and below the ring facilitates this electron delocalization. Any interruption in this conjugation, such as the presence of sp³ hybridized carbon atoms within the ring, breaks the continuity and prevents aromaticity.
Hückel's Rule: The 4n + 2 Electron Count
This rule is the heart of aromaticity. Aromatic compounds must possess (4n + 2) π electrons, where n can be 0, 1, 2, or any non-negative integer. This specific electron count leads to a fully filled set of bonding molecular orbitals, resulting in exceptional stability.
- n = 0: (4(0) + 2) = 2 π electrons (e.g., benzene cation)
- n = 1: (4(1) + 2) = 6 π electrons (e.g., benzene)
- n = 2: (4(2) + 2) = 10 π electrons (e.g., naphthalene)
- n = 3: (4(3) + 2) = 14 π electrons (e.g., anthracene)
Anti-aromaticity: The Unstable Counterpart
Compounds that satisfy the cyclic and planar conditions but possess 4n π electrons are called anti-aromatic. These compounds are exceptionally unstable due to the presence of unpaired electrons in degenerate orbitals. This instability makes anti-aromatic compounds highly reactive. Cyclobutadiene, with 4 π electrons, is a classic example of an anti-aromatic compound.
Non-aromatic Compounds: Lacking the Aromatic Character
Compounds that fail to satisfy one or more of the three criteria for aromaticity are classified as non-aromatic. They are neither exceptionally stable nor exceptionally unstable. Cyclohexane, with its sp³ hybridized carbons and lack of conjugation, is a typical example of a non-aromatic compound.
Identifying Aromatic Ions: A Step-by-Step Approach
Let's consider a hypothetical scenario where we are presented with a series of ions and asked to determine which are aromatic. The following steps will guide us through the process:
- Draw the Lewis structure: Begin by sketching the Lewis structure of each ion, including all lone pairs and formal charges.
- Assess planarity: Determine whether the molecule is planar. Look for any steric hindrance or factors that might distort the planar geometry.
- Identify conjugated system: Check for a continuous system of overlapping p-orbitals encompassing all atoms within the ring. Remember that lone pairs on atoms adjacent to a π-system can participate in conjugation.
- Count π electrons: Count the number of π electrons in the conjugated system. Include electrons from double bonds and lone pairs that are part of the conjugated system.
- Apply Hückel's rule: Determine if the number of π electrons follows the (4n + 2) rule. If so, the ion is aromatic. If the number of π electrons is 4n, the ion is anti-aromatic. If the ion fails to meet the planarity or conjugation criteria, it is non-aromatic.
Examples of Aromatic Ions: A Case Study
Let's analyze specific examples to solidify our understanding. Consider these ions: cyclopentadienyl anion (C₅H₅⁻), cycloheptatrienyl cation (C₇H₇⁺), and cyclooctatetraene dianion (C₈H₈²⁻).
-
Cyclopentadienyl anion (C₅H₅⁻): This anion is planar and cyclic. It has a continuous system of conjugated p-orbitals. The anion contains six π electrons (five from the double bonds and one from the extra electron contributing to the negative charge). Following Hückel's rule (6 = 4n + 2, where n = 1), the cyclopentadienyl anion is aromatic.
-
Cycloheptatrienyl cation (C₇H₇⁺): This cation is planar and cyclic, possesses a continuous system of conjugated p-orbitals, and has six π electrons (from the double bonds, with the positive charge removing an electron from the conjugated pi system). This satisfies Hückel's rule (6 = 4n + 2, where n = 1), making the cycloheptatrienyl cation aromatic.
-
Cyclooctatetraene dianion (C₈H₈²⁻): This dianion is planar (unlike the neutral cyclooctatetraene). It has a continuous system of conjugated p-orbitals. Importantly, it contains ten π electrons (eight from the double bonds and two from the two extra electrons that contribute to the negative charge). Applying Hückel's rule (10 = 4n + 2, where n = 2), the cyclooctatetraene dianion is aromatic.
Conclusion: A Powerful Tool for Predicting Chemical Behavior
Understanding aromaticity is essential for predicting the properties and reactivity of organic molecules. By systematically applying Hückel's rule and considering planarity and conjugation, we can effectively classify cyclic conjugated systems as aromatic, anti-aromatic, or non-aromatic. This knowledge is crucial for various applications, ranging from drug discovery to material science. The ability to accurately identify aromatic ions allows chemists to design and synthesize molecules with desired properties and predict their behavior. This analysis provides a solid framework for further exploration into the fascinating world of aromatic chemistry. Remember that exceptions exist, and further study in advanced organic chemistry may reveal more nuanced situations. However, this foundational understanding provides a solid starting point for comprehending this vital concept.
Latest Posts
Latest Posts
-
A Graph Of The X Component Of The Electric Field
Mar 14, 2025
-
Reactions Which Do Not Continue To Completion Are Called Reactions
Mar 14, 2025
-
How Many Minutes Is Five Hours
Mar 14, 2025
-
Flowering Plants Are Also Known As
Mar 14, 2025
-
Advantages And Disadvantages Of Proprietary Software
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Ions Is Aromatic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
