Which Of The Following Compounds Are Chiral
News Leon
Mar 27, 2025 · 6 min read
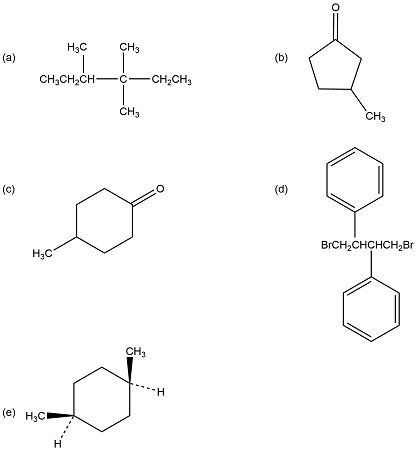
Table of Contents
Which of the following compounds are chiral? A Comprehensive Guide
Chirality, a fundamental concept in organic chemistry, refers to the property of a molecule that is not superimposable on its mirror image. This lack of superimposability is often described as "handedness," analogous to the relationship between your left and right hands. Molecules exhibiting chirality are called chiral, while those that are superimposable on their mirror images are achiral. Understanding chirality is crucial in various fields, including pharmaceuticals, biochemistry, and materials science. This article will delve into the criteria for determining chirality and provide a detailed analysis of how to identify chiral compounds.
Understanding Chirality: The Key Concepts
Before we dive into specific examples, let's solidify our understanding of the core concepts related to chirality. Several factors contribute to a molecule's chirality:
1. The Presence of a Chiral Center (Stereocenter)
The most common cause of chirality is the presence of a chiral center, also known as a stereocenter or stereogenic center. A chiral center is typically a carbon atom bonded to four different substituents. This asymmetry prevents the molecule from being superimposable on its mirror image. While carbon is the most frequent element forming chiral centers, other atoms such as phosphorus, nitrogen, and sulfur can also act as stereocenters under specific circumstances.
2. Absence of a Plane of Symmetry
A crucial characteristic of chiral molecules is the absence of a plane of symmetry. A plane of symmetry divides a molecule into two identical halves that are mirror images of each other. If a molecule possesses a plane of symmetry, it is achiral, regardless of the presence of chiral centers.
3. Enantiomers and Diastereomers
Chiral molecules exist as pairs of enantiomers, which are non-superimposable mirror images. Enantiomers have identical physical properties (e.g., melting point, boiling point) except for their interaction with plane-polarized light and their interaction with other chiral molecules. They rotate plane-polarized light in opposite directions—one clockwise (+), the other counterclockwise (-).
If a molecule possesses more than one chiral center, it can also exhibit diastereomers. Diastereomers are stereoisomers that are not mirror images of each other. Unlike enantiomers, diastereomers often have different physical and chemical properties.
Identifying Chiral Compounds: A Step-by-Step Approach
Let's consider a systematic approach to determining whether a compound is chiral:
-
Draw the molecule: Start by accurately drawing the three-dimensional structure of the molecule. This often involves using wedge and dash notation to represent bonds projecting towards and away from the viewer, respectively.
-
Identify potential chiral centers: Look for carbon atoms (or other atoms) bonded to four different substituents.
-
Check for symmetry: Carefully examine the molecule for any planes of symmetry. If a plane of symmetry exists, the molecule is achiral.
-
Determine if the molecule is superimposable on its mirror image: Draw the mirror image of the molecule and try to superimpose it on the original structure. If they cannot be superimposed, the molecule is chiral.
-
Consider conformational isomers: Be mindful that certain molecules can exist in different conformations (rotated forms) that may or may not be chiral. Consider the most stable conformations to determine chirality.
Examples of Chiral and Achiral Compounds
Let's illustrate the concepts discussed above with specific examples. Consider these compounds:
1. 2-Bromobutane:
This molecule possesses a chiral center at the second carbon atom, which is bonded to four different substituents: a bromine atom, a methyl group, an ethyl group, and a hydrogen atom. It lacks a plane of symmetry and is chiral. It exists as a pair of enantiomers.
2. 1-Bromobutane:
In contrast, 1-bromobutane does not have a chiral center. The first carbon atom is bonded to three hydrogen atoms and a butyl group. It is achiral.
3. 2,3-Dibromobutane:
This molecule has two chiral centers. Depending on the configuration at each center, it can exist as three stereoisomers: two enantiomers and one meso compound. A meso compound is a molecule with chiral centers that possesses a plane of symmetry, making it achiral.
4. 1,2-Dibromocyclohexane:
This molecule has two chiral centers, leading to the possibility of four stereoisomers. However, it is important to note that some of these isomers may have a plane of symmetry (meso forms) and will thus be achiral. Careful analysis of the different configurations is required to determine chirality.
5. Benzene:
Benzene is a completely symmetrical molecule with numerous planes of symmetry. It is therefore achiral.
6. 2,3-Dichlorobutane
This molecule exhibits two chiral centers. With two chiral centers, it can exist as a maximum of 2<sup>n</sup> stereoisomers (where 'n' is the number of chiral centers), which means a maximum of 4. However, due to the possibility of internal symmetry, it possesses only 3 stereoisomers; one meso form and two enantiomers.
7. 1,3-dichlorocyclobutane:
This molecule, seemingly having the potential for chirality based on the substitution pattern, does, in fact, exhibit a plane of symmetry. This makes it achiral. Visualizing the molecule in three dimensions helps identify the plane of symmetry.
8. 2-chloropropanoic acid:
This molecule possesses a chiral center at the second carbon and thus is chiral. It's a key example in illustrating the concepts of enantiomers and optical activity.
9. Ethanal:
Ethanal (acetaldehyde) is a simple aldehyde and is achiral due to its inherent symmetry.
10. Alanine:
Alanine, a common amino acid, contains a chiral center at the alpha-carbon. This chiral center gives rise to two enantiomers: L-alanine and D-alanine, each playing different roles in biological systems. Alanine is thus chiral.
Advanced Considerations and Exceptions
While the presence of a chiral center is a strong indicator of chirality, certain nuances warrant attention:
-
Conformational isomers: Some molecules can interconvert between different conformations that may exhibit different chirality. If the energy barrier between conformations is low, and interconversion is rapid, the molecule may effectively behave as achiral, even if some conformations are chiral.
-
Atropisomers: Atropisomers are stereoisomers that result from hindered rotation around a single bond. This hindered rotation leads to the existence of stable conformers that are non-superimposable mirror images, making them chiral even in the absence of a classical chiral center.
-
Chiral axes and planes: Chirality can arise from elements of molecular symmetry other than chiral centers, such as chiral axes or planes. These are more complex cases requiring advanced analysis.
Conclusion: Mastering Chirality for Success in Chemistry
Determining whether a compound is chiral is a crucial skill in organic chemistry. By understanding the concepts of chiral centers, planes of symmetry, and the different types of stereoisomers, you can confidently analyze molecules and predict their chiral properties. The examples provided offer a practical guide, highlighting the systematic approach to identifying chirality and the subtle variations that can influence this fundamental property. Remember to always visualize the molecule in three dimensions and consider all potential symmetry elements before reaching a conclusion. A deep understanding of chirality is essential for anyone studying or working in organic chemistry, biochemistry, or related fields.
Latest Posts
Latest Posts
-
What Is The Angular Velocity Of The Earth
Mar 30, 2025
-
Which Layer Does Weather Occur In
Mar 30, 2025
-
Economic Growth Is Depicted By
Mar 30, 2025
-
Which Of The Following Is A Conductor
Mar 30, 2025
-
Frame A Sentence Using The Word
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Compounds Are Chiral . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
