Which Molecule Has A Higher Potential Energy
News Leon
Apr 06, 2025 · 5 min read
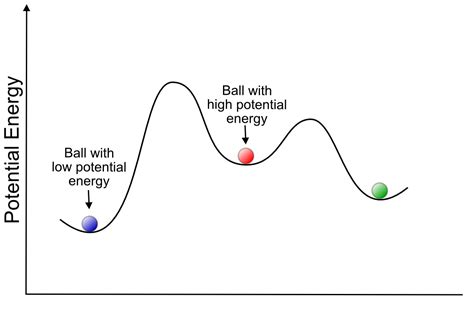
Table of Contents
Which Molecule Has Higher Potential Energy? Understanding Potential Energy in Molecules
Determining which molecule possesses higher potential energy isn't a simple matter of comparing molecular weights or structures. It's a nuanced question that hinges on several factors, primarily the types of bonds present, the molecular geometry, and the overall electronic distribution within the molecule. This article delves deep into the concept of potential energy in molecules, exploring the key elements that influence it and providing examples to illustrate the complexities involved.
Understanding Potential Energy in a Chemical Context
In chemistry, potential energy refers to the stored energy within a molecule due to its structure and the interactions between its constituent atoms. This energy is largely determined by the arrangement of electrons and nuclei and is directly related to the molecule's stability. A molecule with high potential energy is generally less stable and more reactive than a molecule with low potential energy. Think of it like a rock perched precariously on a hilltop – it has high potential energy and is prone to falling (undergoing a reaction). Conversely, a rock at the bottom of the hill has low potential energy and is less likely to move spontaneously.
Several factors contribute to a molecule's potential energy:
1. Bond Strength and Type:
-
Stronger bonds store less potential energy: Strong covalent bonds, such as those found in triple bonds (C≡C) or bonds between highly electronegative atoms (e.g., O=O), have lower potential energy than weaker bonds like single bonds (C-C) or bonds between atoms with significantly different electronegativities (e.g., C-H). Breaking a strong bond requires a substantial input of energy, indicating lower initial potential energy.
-
Bond Length: Shorter bonds generally indicate stronger bonds and thus lower potential energy. Conversely, longer bonds are weaker and possess higher potential energy.
-
Bond polarity: Polar bonds, where electrons are unequally shared between atoms, can have higher potential energy compared to non-polar bonds, due to the electrostatic interactions between the partially charged atoms.
2. Molecular Geometry and Strain:
-
Ring Strain: Cyclic molecules, especially those with small rings (e.g., cyclopropane), experience significant angle strain and torsional strain, leading to higher potential energy compared to their acyclic counterparts or larger ring systems. These strains arise from deviations from ideal bond angles and unfavorable eclipsing interactions between atoms.
-
Steric Hindrance: Bulky groups near each other can cause steric hindrance, leading to increased potential energy due to repulsive interactions. This is commonly observed in branched alkanes compared to their linear isomers.
-
Conformations: Different conformations of a molecule (e.g., staggered vs. eclipsed) can have varying potential energies. Generally, staggered conformations are lower in energy due to reduced steric interactions compared to eclipsed conformations.
3. Electronic Distribution and Resonance:
-
Resonance Structures: Molecules with resonance structures, where electrons can be delocalized across multiple bonds, often possess lower potential energy than molecules without resonance. The delocalization stabilizes the molecule by distributing electron density more evenly. Benzene is a prime example, possessing exceptional stability due to its extensive delocalized π electron system.
-
Electron Density: Uneven electron distribution, such as in polar molecules or molecules with significant charge separation, can lead to higher potential energy due to electrostatic repulsions or attractions.
Comparing Potential Energy: Examples
Let's compare the potential energies of some simple molecules to illustrate these concepts:
Example 1: Ethane vs. Ethene vs. Ethyne
- Ethane (C₂H₆): Contains only single C-C and C-H bonds. It has relatively low potential energy.
- Ethene (C₂H₄): Contains a double C=C bond, which is stronger than a single bond but weaker than a triple bond. It has a slightly higher potential energy than ethane due to the pi-bond.
- Ethyne (C₂H₂): Contains a triple C≡C bond, the strongest of the three. Despite this strong bond, the linear geometry contributes to relatively high energy.
It's important to note that while ethyne has the strongest bond, the overall potential energy comparison is complex and doesn't solely rely on bond strength. Other factors, including bond angles and electron distribution, play crucial roles.
Example 2: Cyclopropane vs. Cyclohexane
- Cyclopropane (C₃H₆): A small ring system with significant angle strain (bond angles are 60° instead of the ideal 109.5° for sp³ hybridized carbon). It has considerably higher potential energy than cyclohexane due to this strain.
- Cyclohexane (C₆H₁₂): A larger ring system that can adopt a chair conformation, minimizing angle strain and torsional strain. It has significantly lower potential energy than cyclopropane.
Example 3: Butane Conformers
Butane exists in various conformations: staggered and eclipsed. The staggered conformations are lower in potential energy due to minimal steric interactions compared to the eclipsed conformations, where methyl groups experience strong repulsion.
Factors Complicating Potential Energy Comparisons
Directly comparing the potential energies of two arbitrary molecules is challenging due to the interplay of multiple factors. While bond strengths provide a general guideline, other effects like molecular geometry, steric interactions, resonance, and electronic effects often dominate. Advanced computational methods, such as density functional theory (DFT) and molecular mechanics calculations, are often employed to accurately determine and compare the potential energies of molecules.
Predicting Relative Potential Energies: A Practical Approach
While precise calculations require advanced techniques, you can often make reasonable predictions about relative potential energies using these guidelines:
- Consider bond strengths: Stronger bonds generally imply lower potential energy. Prioritize triple bonds over double bonds, and double bonds over single bonds.
- Assess ring strain: Small rings have high potential energy due to angle and torsional strain.
- Evaluate steric hindrance: Bulky groups near each other lead to higher potential energy.
- Look for resonance: Delocalized electrons stabilize the molecule, lowering potential energy.
- Analyze bond polarity: Polar bonds can contribute to higher potential energy due to electrostatic interactions.
By systematically considering these factors, you can often make educated estimations of relative potential energies for different molecules, even without advanced computational tools.
Conclusion: A Multifaceted Property
Determining which molecule has higher potential energy is a complex issue that necessitates a holistic understanding of molecular structure and bonding. While bond strengths provide a fundamental basis for comparison, other factors like ring strain, steric hindrance, resonance, and electronic effects play equally critical roles. Understanding these factors allows for more informed predictions and interpretations of molecular behavior and reactivity. Advanced computational methods are invaluable tools for more accurate assessments, but the principles outlined in this article offer a practical approach to making informed comparisons. Remember that the potential energy of a molecule isn't simply a single number but a reflection of its intricate internal structure and interactions.
Latest Posts
Latest Posts
-
What Is The Molar Mass Of H2o2
Apr 08, 2025
-
Female Flowers In Cucumber Is Increased By Spraying Of
Apr 08, 2025
-
Is Sound Wave A Mechanical Wave
Apr 08, 2025
-
Draw The Monomer For The Following Polymer
Apr 08, 2025
-
What Is The Primary Source Of Energy On Earth
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Which Molecule Has A Higher Potential Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.