Which Is A Characteristic Of Mixtures
News Leon
Mar 17, 2025 · 8 min read
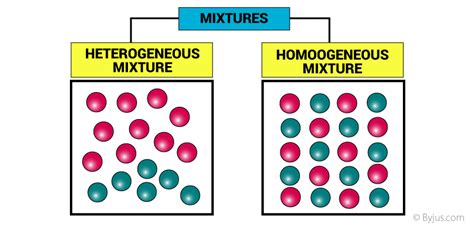
Table of Contents
Which is a Characteristic of Mixtures? A Deep Dive into the Properties of Mixtures
Mixtures are everywhere! From the air we breathe to the food we eat, mixtures are a fundamental part of our world. But what exactly is a mixture, and what characteristics define them? This comprehensive guide delves into the fascinating world of mixtures, exploring their properties and distinguishing them from pure substances. We'll cover various types of mixtures, their unique characteristics, and their importance in various fields.
Understanding Mixtures: A Definition
A mixture is a substance composed of two or more components not chemically bonded. A key characteristic is that the components retain their individual chemical properties. This means you can, in principle, separate the components of a mixture using physical methods like filtration, distillation, or evaporation. This contrasts sharply with compounds, where the components have reacted chemically to form a new substance with different properties.
Think of making a salad: you combine lettuce, tomatoes, cucumbers, and dressing. Each ingredient retains its identity; you can easily pick out the lettuce leaves from the tomatoes. This is a classic example of a mixture.
Key Characteristics of Mixtures
Several characteristics distinguish mixtures from pure substances. These include:
1. Variable Composition:
One of the most defining features of a mixture is its variable composition. Unlike compounds, which have a fixed ratio of elements, mixtures can have varying proportions of their components. For example, you can make a weak cup of tea or a strong one—both are still tea mixtures, but their concentrations of tea leaves differ. This flexibility in composition is a hallmark of mixtures.
2. Retention of Individual Properties:
The components of a mixture retain their individual physical and chemical properties. In our salad example, the lettuce remains green and crisp, the tomatoes remain red and juicy, even when combined. This contrasts with compounds where the resulting substance has entirely new properties. Salt (NaCl), for example, is vastly different from its constituent elements, sodium (a highly reactive metal) and chlorine (a toxic gas).
3. No Chemical Changes:
The formation of a mixture does not involve chemical changes. No new substances are formed; the components simply mix together. This is crucial in distinguishing mixtures from chemical reactions, where new substances with different properties are created.
4. Separable Components:
The components of a mixture can be separated by physical means. This is a powerful way to identify a mixture. Techniques like filtration (separating solids from liquids), distillation (separating liquids with different boiling points), chromatography (separating components based on their differing affinities for a stationary and mobile phase), evaporation (separating a dissolved solid from a liquid), and decantation (separating immiscible liquids) can all be used to separate the components of a mixture.
5. Homogeneous vs. Heterogeneous:
Mixtures are categorized into two main types:
Homogeneous Mixtures:
Also known as solutions, these mixtures have a uniform composition throughout. The components are evenly distributed at a molecular level, making it impossible to visually distinguish them. Examples include saltwater, air, and sugar dissolved in water. Even though they are composed of multiple components, they appear as a single phase.
Heterogeneous Mixtures:
These mixtures have a non-uniform composition. The different components are visible and can be easily distinguished. Examples include sand and water, oil and water, and a salad. These mixtures often consist of two or more distinct phases.
Types of Mixtures: A Detailed Exploration
Let's delve deeper into the various types of mixtures, illustrating their properties and applications:
1. Solutions:
Solutions are homogeneous mixtures where one substance (the solute) is dissolved uniformly in another substance (the solvent). The solute particles are so small they are invisible to the naked eye, and the solution appears to be a single phase. Examples:
- Solid dissolved in liquid: Saltwater (salt is the solute, water is the solvent)
- Liquid dissolved in liquid: Alcohol in water
- Gas dissolved in liquid: Carbonated water (carbon dioxide dissolved in water)
- Gas dissolved in gas: Air (oxygen, nitrogen, and other gases)
The properties of a solution are often different from the properties of its individual components. For instance, saltwater freezes at a lower temperature than pure water. The concentration of a solution (the amount of solute dissolved in a given amount of solvent) is an important property, often expressed as molarity or percentage by mass.
2. Suspensions:
Suspensions are heterogeneous mixtures where solid particles are dispersed in a liquid or gas. The particles are relatively large and tend to settle out over time if left undisturbed. Examples:
- Muddy water: Soil particles suspended in water
- Dust in air: Solid dust particles dispersed in air
Suspensions can be separated by simple filtration, as the solid particles are large enough to be trapped by filter paper.
3. Colloids:
Colloids are heterogeneous mixtures where particles are intermediate in size between those in solutions and suspensions. These particles are dispersed throughout a medium but do not settle out easily. The particles scatter light, giving colloids a cloudy or opaque appearance (Tyndall effect). Examples:
- Milk: Fat globules dispersed in water
- Fog: Water droplets suspended in air
- Mayonnaise: Oil droplets emulsified in water
Colloids are often stabilized by emulsifiers, which prevent the particles from separating.
4. Alloys:
Alloys are homogeneous mixtures of two or more metals. The properties of an alloy are often different from the properties of its constituent metals. For example, bronze (a mixture of copper and tin) is stronger and more durable than either copper or tin alone. Steel, a mixture of iron and carbon, is another common example. The specific properties of an alloy are highly dependent on the proportions of its constituent metals.
5. Emulsions:
Emulsions are heterogeneous mixtures of two or more immiscible liquids. One liquid is dispersed as droplets within another liquid. Emulsions require an emulsifier to prevent the liquids from separating. Examples:
- Mayonnaise: Oil droplets dispersed in water, stabilized by egg yolk
- Milk: Fat globules dispersed in water
Separating Mixtures: Techniques and Applications
The ability to separate mixtures is crucial in many scientific and industrial processes. The choice of separation technique depends on the type of mixture and the properties of its components.
1. Filtration:
Used to separate solids from liquids. The mixture is passed through a filter paper, which traps the solid particles while allowing the liquid to pass through. This technique is commonly used in water purification and in separating precipitates in chemical reactions.
2. Distillation:
Separates liquids with different boiling points. The mixture is heated, and the liquid with the lower boiling point vaporizes first, is condensed, and collected separately. This method is widely used in the production of alcoholic beverages and in refining petroleum.
3. Chromatography:
Separates components based on their different affinities for a stationary and mobile phase. The mixture is passed through a column or spread across a plate, and the components separate based on their interactions with the stationary and mobile phases. Chromatography is a powerful technique used in analytical chemistry and biochemistry.
4. Evaporation:
Separates a dissolved solid from a liquid by evaporating the liquid, leaving the solid behind. This is a simple and effective method for obtaining salts from saltwater.
5. Decantation:
Separates immiscible liquids by carefully pouring off the top layer. This technique is often used to separate oil and water.
6. Magnetism:
Separates magnetic materials from non-magnetic materials using a magnet. This method is often used to remove iron filings from a mixture.
7. Centrifugation:
Separates components based on their density. The mixture is spun at high speed, causing denser components to settle at the bottom while lighter components remain at the top. Centrifugation is used in various applications, including blood separation and separating sediment from liquids.
The Importance of Mixtures in Everyday Life and Industry
Mixtures are integral to our daily lives and play a significant role in various industries. From the air we breathe to the food we eat, mixtures are omnipresent. Their importance is evident in diverse sectors:
-
Food Industry: Many food products are mixtures, such as sauces, soups, and beverages. Understanding the properties of these mixtures is crucial for maintaining quality and ensuring food safety.
-
Pharmaceutical Industry: Many medications are mixtures of active ingredients and inactive excipients. Careful control of mixture properties is vital for drug efficacy and safety.
-
Materials Science: Alloys, composites, and other materials are mixtures with tailored properties for specific applications.
-
Environmental Science: Understanding the composition and properties of environmental mixtures is essential for pollution control and environmental monitoring.
-
Chemical Industry: Many chemical processes involve mixing and separating different substances. Effective mixing and separation techniques are crucial for efficient and safe chemical production.
Conclusion: The Versatile World of Mixtures
Mixtures are ubiquitous in our world, exhibiting a fascinating array of properties and applications. Their variable composition, retention of individual properties, and separability by physical means are key defining characteristics. Understanding the different types of mixtures, their unique properties, and the techniques for separating them is vital in various scientific and industrial fields. This knowledge empowers us to harness the versatility of mixtures for innovative applications and to tackle complex challenges across many disciplines. Further exploration into the specific properties of different mixtures opens up avenues for ongoing research and development.
Latest Posts
Latest Posts
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Is A Characteristic Of Mixtures . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
