Which Functional Group Is Found In Methyl Ethanoate
News Leon
Mar 23, 2025 · 5 min read
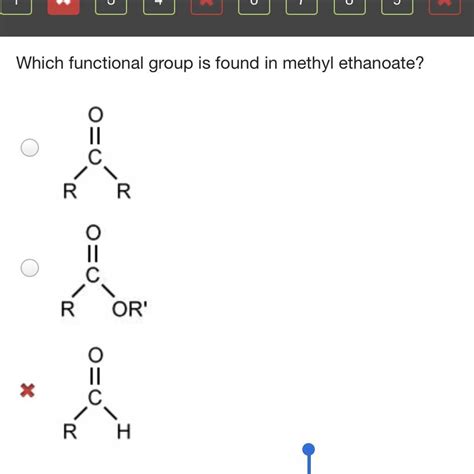
Table of Contents
Which Functional Group is Found in Methyl Ethanoate? An In-Depth Exploration
Methyl ethanoate, also known as methyl acetate, is a simple ester commonly used as a solvent. Understanding its chemical structure, specifically identifying its functional group, is crucial for comprehending its properties and reactivity. This article delves deep into the structure of methyl ethanoate, pinpointing its functional group, explaining its characteristics, and exploring related concepts in organic chemistry.
Identifying the Functional Group: The Ester
The key to understanding methyl ethanoate lies in recognizing its functional group: the ester group. This is where the magic happens, dictating the compound's unique chemical behavior. The ester functional group is characterized by a carbonyl group (C=O) bonded to an oxygen atom (O), which is further bonded to a carbon atom. This can be represented as -COO-.
Visualizing the Structure
To fully grasp this, let's visualize the structural formula of methyl ethanoate:
O
||
CH3-C-O-CH3
Here, we clearly see the ester functional group (-COO-) at the heart of the molecule. The carbonyl carbon (C=O) is bonded to a methyl group (CH3) on one side and an oxygen atom bonded to another methyl group (CH3) on the other.
Comparing to other Functional Groups
It's important to differentiate the ester functional group from other similar groups like:
-
Carboxylic Acids (-COOH): These contain a carbonyl group and a hydroxyl group (-OH) attached to the same carbon. Methyl ethanoate is derived from a carboxylic acid (acetic acid), but the hydroxyl group is replaced by an alkoxy group (-OR). This key difference results in distinct properties. Carboxylic acids are acidic, while esters are generally neutral.
-
Ketones (R-CO-R'): Ketones also contain a carbonyl group, but it's bonded to two carbon atoms, not an oxygen and a carbon like in esters. Ketones and esters show different chemical reactivities.
-
Aldehydes (R-CHO): Similar to ketones, aldehydes also have a carbonyl group, but it’s bonded to one carbon atom and one hydrogen atom. Again, their chemical behavior vastly differs from esters.
-
Ethers (R-O-R'): Ethers contain an oxygen atom bonded to two carbon atoms, but they lack the carbonyl group (C=O) characteristic of esters.
Properties Influenced by the Ester Functional Group
The ester functional group is responsible for many of the characteristic properties of methyl ethanoate:
Odor and Flavor
Esters are known for their pleasant aromas and fruity flavors. Methyl ethanoate, in particular, has a sweet, somewhat solvent-like odor. Many natural fruit flavors and fragrances contain esters. This is a crucial aspect of their use in food and perfume industries. The specific scent varies depending on the structure of the R groups attached to the ester functional group.
Volatility
Methyl ethanoate is a volatile liquid, meaning it easily evaporates at room temperature. This volatility is partially due to the relatively weak intermolecular forces between ester molecules. The absence of strong hydrogen bonding, unlike in carboxylic acids, contributes significantly to this characteristic. Volatility is important for its applications as a solvent.
Solubility
The solubility of methyl ethanoate in water is moderate. While the ester group can participate in weak hydrogen bonding with water molecules, the nonpolar alkyl groups reduce its overall water solubility. It’s more soluble in organic solvents due to the non-polar nature of the alkyl groups.
Chemical Reactions of Methyl Ethanoate: The Ester Group in Action
The ester functional group is the reactive center in methyl ethanoate. It participates in several important reactions, including:
Hydrolysis (Acidic and Basic)
Hydrolysis involves breaking the ester bond with the addition of water. Acidic hydrolysis produces the carboxylic acid (acetic acid) and alcohol (methanol). Basic hydrolysis (saponification) yields the carboxylate salt and alcohol. These reactions are reversible. The choice between acidic or basic hydrolysis often depends on the desired products and reaction conditions.
Transesterification
This reaction involves the exchange of the alkoxy group (-OR) of one ester with another alcohol. This reaction is catalyzed by acids or bases and is widely employed in the synthesis of various esters and biodiesel production. This highlights the functional role of the ester group in exchanging parts of the molecule.
Reduction
Esters can be reduced to alcohols using reducing agents like lithium aluminum hydride (LiAlH4). This reaction breaks the ester bond and converts the carbonyl group to a hydroxyl group. This reaction allows for modifying the structure of esters to obtain alcohols, altering their properties.
Synthesis of Methyl Ethanoate: Formation of the Ester Group
The ester functional group in methyl ethanoate is formed through a reaction called esterification. This usually involves reacting a carboxylic acid (acetic acid) with an alcohol (methanol) in the presence of an acid catalyst (such as sulfuric acid). The reaction is reversible, and the equilibrium is shifted toward ester formation by removing the water produced.
Applications Leveraging the Ester Functional Group
The unique properties of methyl ethanoate, stemming from its ester functional group, lead to diverse applications:
-
Solvent: Its volatility and moderate polarity make it an excellent solvent in various industries, including paints, coatings, inks, and adhesives.
-
Chemical Intermediate: Methyl ethanoate serves as a building block for the synthesis of other chemicals, including pharmaceuticals and polymers.
-
Flavor and Fragrance: Its pleasant odor makes it useful in food and cosmetic industries to add fruity notes.
-
Extraction: Its solubility characteristics make it a useful solvent for extracting certain compounds.
Conclusion: The Ester Group's Central Role
In summary, the ester functional group (-COO-) is the defining characteristic of methyl ethanoate. Its presence dictates the compound's unique properties, reactivity, and subsequent applications. Understanding the structure and reactions of this functional group is essential in organic chemistry and various related fields. The ester group's ability to participate in various reactions like hydrolysis and transesterification, coupled with its pleasant odor and useful solvent properties, makes methyl ethanoate a versatile compound with significant industrial relevance. Further exploration of ester chemistry reveals its significant role in diverse fields, ranging from pharmaceuticals to materials science. This detailed analysis highlights the importance of understanding functional groups in comprehending the properties and behaviors of organic molecules.
Latest Posts
Latest Posts
-
Lcm Of 5 10 And 15
Mar 25, 2025
-
A Battleship Simultaneously Fires Two Shells
Mar 25, 2025
-
Whats The Difference Between Rough And Smooth Er
Mar 25, 2025
-
The Current In An Rl Circuit Builds Up To One Third
Mar 25, 2025
-
Which Shape Does Not Have A Line Of Symmetry
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which Functional Group Is Found In Methyl Ethanoate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
