Where Is Hydrogen In The Periodic Table
News Leon
Mar 23, 2025 · 5 min read
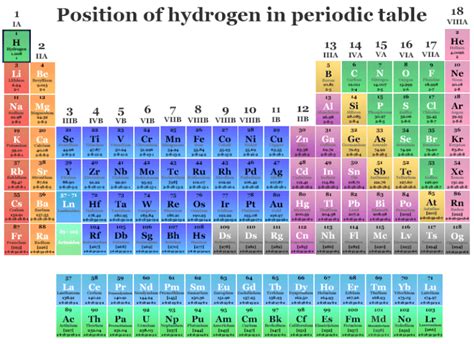
Table of Contents
Where is Hydrogen in the Periodic Table? Unpacking the Unique Element
Hydrogen, the simplest and most abundant element in the universe, holds a unique position in the periodic table. Its placement is a source of ongoing discussion and highlights its unusual chemical properties. While often placed at the top of Group 1 (alkali metals), its behavior sometimes aligns more closely with Group 17 (halogens). This article will delve deep into the rationale behind its positioning, exploring its properties and comparing it to its neighboring groups to fully understand its unique place in the periodic table.
The Periodic Table: A System of Organization
Before we pinpoint hydrogen's location, let's briefly revisit the organization of the periodic table. Developed by Dmitri Mendeleev in the late 19th century, the periodic table arranges elements based on their atomic number (number of protons), electronic configuration, and recurring chemical properties. Elements are organized into rows (periods) and columns (groups) reflecting these patterns.
Groups represent elements with similar outer electron configurations, leading to similar chemical behaviors. For example, Group 1 elements (alkali metals) all have one electron in their outermost shell, making them highly reactive. Group 17 (halogens) possess seven electrons in their outermost shell, exhibiting high electronegativity and a tendency to gain an electron to achieve a stable octet.
Hydrogen's Placement: A Matter of Debate
Hydrogen's placement is a subject of ongoing discussion because it doesn't perfectly fit into any single group. Its atomic number is 1, possessing only one proton and one electron. This simple electronic structure is what makes it so unique.
Argument for Group 1 (Alkali Metals):
- Single Valence Electron: Like alkali metals, hydrogen has a single electron in its outermost shell. This leads to a tendency to lose this electron to form a +1 cation (H+), similar to the alkali metals forming +1 ions like Na+ and K+.
- Similar Reactivity (in some cases): Hydrogen can react with nonmetals to form covalent compounds, similar to the reactivity of alkali metals with nonmetals. For instance, hydrogen reacts with chlorine to form hydrogen chloride (HCl), mirroring the reaction of sodium with chlorine to form sodium chloride (NaCl).
Argument Against Group 1 (Alkali Metals):
- Non-Metallic Character: Unlike alkali metals, hydrogen is a nonmetal gas under standard conditions. Alkali metals are soft, silvery-white metals with low ionization energies. Hydrogen shows significantly different physical properties.
- Covalent Bonding Preference: While it can lose an electron, hydrogen more readily shares its electron in covalent bonding. Alkali metals primarily form ionic compounds through electron transfer.
Argument for Group 17 (Halogens):
- Electron Affinity: Hydrogen possesses a relatively high electron affinity, meaning it has a strong tendency to gain an electron. This is a characteristic feature of halogens, which readily gain one electron to form a -1 anion (like Cl-).
- Diatomic Nature: Hydrogen exists as a diatomic molecule (H₂), similar to the halogens which exist as diatomic molecules (like Cl₂, F₂). This diatomic nature arises from the tendency to achieve a stable electron configuration.
Argument Against Group 17 (Halogens):
- Low Electronegativity Compared to Halogens: While hydrogen can gain an electron, its electronegativity is considerably lower than that of halogens. Fluorine, the most electronegative element, has a significantly higher electronegativity than hydrogen.
- Different Reactivity Patterns: While sharing a diatomic nature with halogens, hydrogen displays vastly different reactivity patterns compared to halogens. Its reactions are often less vigorous and selective than those of halogens.
The Compromise: A Unique Position Above the Table
Due to its unique characteristics, hydrogen is typically placed separately at the top left of the periodic table, above the alkali metals. This positioning acknowledges its single valence electron while emphasizing its distinct physical and chemical properties differentiating it from both Group 1 and Group 17. This separate placement visually reflects its unique status as an element unlike any other.
Hydrogen's Isotopes: Further Complicating the Picture
The complexity of hydrogen's position is further heightened by the existence of its isotopes: protium (¹H), deuterium (²H or D), and tritium (³H or T). These isotopes differ in the number of neutrons in their nucleus, leading to slight variations in their physical properties. While these variations don't significantly alter their placement on the periodic table, they highlight the intricate nature of this unique element.
Understanding Hydrogen's Role in Chemistry and Beyond
Hydrogen's unusual properties significantly impact its role in chemistry and various applications:
- Acid-Base Chemistry: The H+ ion is crucial in acid-base reactions, playing a vital role in defining acidity and pH.
- Organic Chemistry: Hydrogen is a fundamental component of organic molecules, forming the backbone of countless compounds.
- Energy Production: Hydrogen is being explored as a clean energy source, offering the potential for a sustainable future through fuel cells and other applications.
- Industrial Processes: Hydrogen is used extensively in various industrial processes, including ammonia production and metal refining.
Conclusion: Hydrogen's Enduring Enigma
Hydrogen's position on the periodic table continues to spark debate, underscoring its unique and somewhat anomalous properties. While its single valence electron links it to the alkali metals, its non-metallic character and ability to form covalent bonds distinguish it significantly. Its placement above the alkali metals reflects a compromise, recognizing its similarities and differences while highlighting its exceptional nature. Its unique properties make it a fascinating element with applications that span countless fields, making its study vital in understanding the fundamental laws governing the behavior of matter. Further research continues to unveil the complexities of this essential element, reinforcing its unique and irreplaceable position in the universe and within the intricate structure of the periodic table.
Latest Posts
Latest Posts
-
How Many Hours Is In 3 Weeks
Mar 25, 2025
-
The Y Axis Of A Velocity Time Graph Represents
Mar 25, 2025
-
How Many Moles In 22g Of Co2
Mar 25, 2025
-
A Toiroidal Solenoid Has A Central Radius Of 0 5m
Mar 25, 2025
-
Why Blood Is A Connective Tissue
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Where Is Hydrogen In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
