Where Does Oxidation Take Place In An Electrochemical Cell
News Leon
Mar 24, 2025 · 6 min read
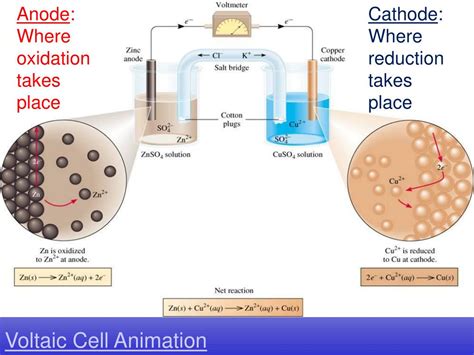
Table of Contents
Where Does Oxidation Take Place in an Electrochemical Cell? A Deep Dive into Redox Reactions
Electrochemical cells are fascinating devices that harness the power of redox reactions to generate electricity or drive chemical changes. Understanding where oxidation and reduction occur within these cells is fundamental to grasping their operation. This article delves deep into the specifics of oxidation within electrochemical cells, exploring different cell types and the crucial role of electrodes.
Understanding Oxidation and Reduction
Before we pinpoint the location of oxidation in an electrochemical cell, let's establish a clear understanding of the fundamental concepts of oxidation and reduction. These processes, collectively known as redox reactions, always occur simultaneously.
-
Oxidation: This involves the loss of electrons by a chemical species. The species undergoing oxidation is called the reducing agent because it donates electrons to another species. Common indicators of oxidation include an increase in oxidation state (a positive charge) and the formation of oxygen-containing compounds.
-
Reduction: This involves the gain of electrons by a chemical species. The species undergoing reduction is called the oxidizing agent because it accepts electrons from another species. Common indicators of reduction include a decrease in oxidation state (a negative charge) and the formation of hydrogen-containing compounds.
The mnemonic OIL RIG (Oxidation Is Loss, Reduction Is Gain) is often used to remember these definitions.
Types of Electrochemical Cells
Electrochemical cells are broadly categorized into two main types:
1. Galvanic Cells (Voltaic Cells)
These cells generate electricity spontaneously through a redox reaction. The chemical energy stored within the reactants is converted into electrical energy. A classic example is the Daniell cell, which uses zinc and copper electrodes.
2. Electrolytic Cells
These cells require an external source of electrical energy to drive a non-spontaneous redox reaction. Electricity is used to force a chemical change to occur. Electroplating and the production of aluminum are common applications of electrolytic cells.
The Anatomy of an Electrochemical Cell
Regardless of the cell type, several key components are essential:
-
Electrodes: These are conductive materials (typically metals or graphite) that serve as the sites where oxidation and reduction occur. There are two electrodes: the anode and the cathode.
-
Electrolyte: This is an ionic conductor, usually a solution or molten salt, that allows the flow of ions between the electrodes. It completes the electrical circuit by transporting charged particles.
-
Salt Bridge (for Galvanic Cells): This is a U-shaped tube filled with an electrolyte solution that connects the two half-cells in a galvanic cell. It prevents the buildup of charge imbalance and maintains electrical neutrality.
Where Oxidation Takes Place: The Anode
The crucial answer to our central question lies in understanding the role of the anode: Oxidation always takes place at the anode. This is true for both galvanic and electrolytic cells.
At the anode, the reducing agent loses electrons, thereby undergoing oxidation. These electrons then flow through the external circuit towards the cathode. The anode is often negatively charged in galvanic cells (as electrons accumulate) and positively charged in electrolytic cells (as electrons are drawn away).
Oxidation at the Anode: A Closer Look
Let's examine the specifics of oxidation at the anode in different scenarios:
In a Galvanic Cell: The anode is the site where the more active metal (the one with a higher tendency to lose electrons) undergoes oxidation. For example, in the Daniell cell, the zinc electrode (anode) oxidizes:
Zn(s) → Zn²⁺(aq) + 2e⁻
The released electrons flow through the external circuit to the copper cathode.
In an Electrolytic Cell: The anode is the site where oxidation of the electrolyte's anions or the electrode material itself occurs, depending on the electrochemical potentials involved. For example, in the electrolysis of water, the anode is typically made of an inert material like platinum. Water molecules are oxidized:
2H₂O(l) → O₂(g) + 4H⁺(aq) + 4e⁻
This produces oxygen gas at the anode. In other electrolytic processes, the anode itself might oxidize, gradually dissolving into the electrolyte.
Distinguishing Between Anode and Cathode
It's crucial to remember that the assignment of anode and cathode is based on the process occurring at each electrode, not on the electrode's charge. While the anode is often negatively charged in a galvanic cell and positively charged in an electrolytic cell, the defining characteristic of the anode is that it is the site of oxidation.
This distinction highlights the importance of understanding the underlying redox reaction rather than simply relying on charge as the primary identifier. The terms anode and cathode are relative to the specific electrochemical process occurring within the cell.
Factors Influencing Oxidation at the Anode
Several factors can influence the rate and efficiency of oxidation at the anode:
-
Electrode Material: The choice of electrode material significantly impacts the ease with which oxidation occurs. Inert electrodes like platinum or graphite are often used in situations where the oxidation of the electrode itself is undesirable.
-
Electrolyte Concentration: The concentration of the species being oxidized influences the rate of the reaction. Higher concentrations generally lead to faster oxidation.
-
Temperature: Increasing temperature usually accelerates the rate of oxidation.
-
Surface Area: A larger surface area of the anode increases the contact area between the electrode and the electrolyte, promoting faster oxidation.
-
Presence of Catalysts: Catalysts can significantly enhance the rate of the oxidation reaction by lowering the activation energy.
Practical Applications: Highlighting Oxidation at the Anode
Understanding where oxidation takes place is crucial in various practical applications of electrochemical cells.
1. Batteries: In batteries (a type of galvanic cell), the anode is where the chemical energy is released through oxidation. This releases electrons that travel through the circuit, powering devices. The specific materials used for the anode determine the battery's voltage and lifespan.
2. Fuel Cells: Fuel cells, also galvanic cells, use oxidation of a fuel (often hydrogen) at the anode to generate electricity. The efficiency and performance of fuel cells heavily depend on the anode's catalytic activity and design.
3. Corrosion: Corrosion of metals is essentially an electrochemical process where oxidation at the anode leads to the deterioration of the metal. Understanding this process allows for the development of corrosion-resistant materials and protective coatings.
4. Electroplating: In electroplating (an electrolytic process), the anode is where the metal to be plated is oxidized, releasing metal ions into the electrolyte solution. These ions then deposit onto the cathode, covering the object to be plated.
5. Chlor-Alkali Process: This industrial process uses an electrolytic cell to produce chlorine gas and sodium hydroxide. At the anode, chloride ions are oxidized, generating chlorine gas.
Conclusion: The Significance of the Anode in Electrochemical Processes
The anode, as the site of oxidation in electrochemical cells, plays a pivotal role in determining the cell's function and efficiency. Its material, surface area, and the specific redox reactions occurring at it directly impact the cell's performance, whether it's generating electricity in a battery or driving a chemical change in an electrolytic cell. Understanding the intricacies of oxidation at the anode is crucial for designing, optimizing, and utilizing electrochemical technologies effectively across various scientific and engineering disciplines. Further research into materials science, electrocatalysis, and reaction kinetics continues to refine our understanding and unlock new possibilities for advancements in electrochemical technologies. The ongoing exploration of anode materials and their interaction with electrolytes promises exciting developments in energy storage, energy conversion, and other applications.
Latest Posts
Latest Posts
-
How Many Different Phone Numbers Are Possible
Mar 29, 2025
-
What Day Will It Be In 150 Days
Mar 29, 2025
-
Black Fur In Mice Is Dominant To Brown Fur
Mar 29, 2025
-
Occupies Space Between Cell Membrane And Nucleus
Mar 29, 2025
-
Give A Reason For Your Answer
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Where Does Oxidation Take Place In An Electrochemical Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
