When Group 2a Elements Form Ions They
News Leon
Apr 06, 2025 · 7 min read
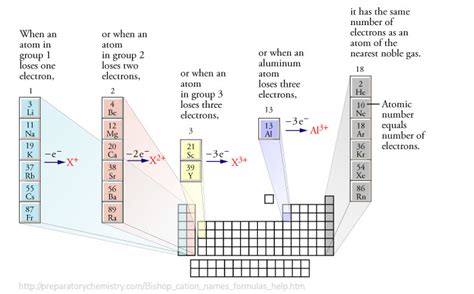
Table of Contents
When Group 2A Elements Form Ions: A Deep Dive into Alkaline Earth Metal Chemistry
Group 2A elements, also known as alkaline earth metals, are fascinating subjects in chemistry, primarily due to their predictable and consistent behavior when forming ions. Understanding their ionic formation is crucial for grasping various chemical processes and applications. This comprehensive article delves deep into the ionic characteristics of Group 2A elements, covering their electron configuration, ionization energies, ionic radii, and the properties of their resulting compounds.
Electronic Configuration and Ionization: The Foundation of Ion Formation
Alkaline earth metals—beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra)—share a common feature: they possess two electrons in their outermost s orbital. This electronic configuration, represented as [noble gas]ns², dictates their chemical reactivity and ion formation. The [noble gas] represents the electronic configuration of the preceding noble gas in the periodic table. For example, magnesium ([Ne]3s²) has the same inner electron configuration as neon.
The tendency to achieve a stable noble gas configuration drives the chemical behavior of these elements. To achieve this stable octet, they readily lose their two valence electrons, resulting in the formation of divalent cations with a +2 charge. This process is known as ionization.
Ionization Energies: The Energy Cost of Ion Formation
The energy required to remove an electron from a neutral atom is called the ionization energy. Group 2A elements have relatively low first ionization energies compared to other groups, reflecting the ease with which they lose their valence electrons. However, their second ionization energies are significantly higher than their first, demonstrating the increasing difficulty of removing electrons from increasingly positively charged ions. This is because after losing one electron, the remaining electron is more strongly attracted to the positively charged ion.
The trend in first ionization energy within Group 2A shows a general decrease down the group. This is because as you move down the group, the atomic radius increases. The increased distance between the nucleus and the valence electrons results in a weaker electrostatic attraction, making it easier to remove the electrons.
Comparing Ionization Energies Across Periods: Why the Difference?
It's important to compare Group 2A ionization energies to those of other groups. For example, Group 1A elements (alkali metals) have even lower first ionization energies because they only need to lose one electron to achieve a noble gas configuration. Conversely, elements in Group 13 (boron group) have higher first ionization energies because they need to lose three electrons, a significantly more energy-intensive process. This difference directly impacts the reactivity of these elements.
Ionic Radii: The Size Matters
Once the alkaline earth metals lose their two valence electrons and become divalent cations, their ionic radii become considerably smaller than their atomic radii. This is because the removal of the outermost electron shell reduces the overall size of the ion.
The ionic radius increases down Group 2A. This is a direct consequence of the increased number of electron shells. The additional electrons and shells shield the outer electrons from the nuclear charge, leading to larger ionic sizes.
The Impact of Ionic Radius on Properties
The ionic radius plays a crucial role in determining the properties of ionic compounds formed by alkaline earth metals. Smaller ions generally lead to stronger ionic bonds due to the greater electrostatic attraction between the cation and the anion. This influences the melting and boiling points, solubility, and other physical properties of these compounds.
Properties of Group 2A Compounds: A Result of Ionic Bonding
The divalent cations formed by Group 2A elements readily react with various anions to form a wide range of ionic compounds. These compounds exhibit several characteristic properties largely determined by the strong electrostatic forces between the ions:
-
High melting and boiling points: The strong electrostatic attraction between the positively charged cations and negatively charged anions requires a large amount of energy to overcome, resulting in high melting and boiling points. This is especially true for compounds formed with smaller anions.
-
Crystalline structure: Ionic compounds of Group 2A elements typically crystallize in well-defined lattice structures due to the ordered arrangement of ions to maximize electrostatic attractions and minimize repulsions.
-
Solubility in water: The solubility of Group 2A compounds varies. Generally, compounds with smaller anions (like fluorides and oxides) are less soluble than those with larger anions (like chlorides, bromides, and iodides). Solubility also increases down the group as the ionic radius increases, weakening the lattice energy.
-
Hardness and brittleness: Ionic compounds are often hard and brittle due to the strong ionic bonds. However, the exact hardness and brittleness depend on the size and charge of the ions involved.
Specific Examples: A Closer Look at Individual Elements
Let's examine the ionic behavior of some individual Group 2A elements in greater detail:
Magnesium (Mg): Essential for Life
Magnesium readily forms Mg²⁺ ions. Magnesium ions play a vital role in biological systems, acting as cofactors for numerous enzymes. Compounds like magnesium oxide (MgO), a key component of refractory materials, and magnesium hydroxide (Mg(OH)₂), used as an antacid, demonstrate the diverse applications of magnesium compounds.
Calcium (Ca): Bones and Beyond
Calcium (Ca²⁺) is crucial for bone health and various biological processes. Calcium carbonate (CaCO₃) is the primary component of limestone, marble, and chalk, exhibiting various industrial and construction applications. Calcium sulfate (CaSO₄), or gypsum, is used in plaster and drywall.
Beryllium (Be): A Unique Case
Beryllium is an exception among alkaline earth metals due to its small size and high charge density. It forms Be²⁺ ions, but its chemistry is often complicated by its tendency to form covalent bonds as well, particularly with highly electronegative elements such as oxygen and fluorine. This difference in behavior is largely attributed to the very high ionization energy of beryllium compared to other Group 2A elements.
Strontium, Barium, and Radium: Increasing Reactivity
As we move down the group to strontium (Sr), barium (Ba), and radium (Ra), the reactivity of the elements increases due to the decreasing ionization energies and increasing atomic radii. Their compounds find applications in various areas, such as pyrotechnics (strontium salts produce red flames) and medical imaging (barium sulfate is used as a contrast agent). Radium, however, is radioactive and poses significant health risks.
Applications of Group 2A Compounds: A Wide Range of Uses
The compounds formed by Group 2A elements find numerous applications in various industries:
-
Construction and building materials: Calcium carbonate (limestone) is a fundamental building material in cement and concrete. Gypsum (calcium sulfate) is used in plaster and drywall.
-
Industrial processes: Magnesium oxide is used as a refractory material due to its high melting point. Calcium oxide (lime) is used in steelmaking and other industrial processes.
-
Medicine: Magnesium and calcium ions are vital in biological systems, and their compounds are used in antacids and other medications. Barium sulfate is used as a contrast agent in medical imaging.
-
Agriculture: Calcium and magnesium are essential nutrients for plant growth, and their compounds are used in fertilizers.
-
Pyrotechnics: Strontium salts are used to produce red flames in fireworks, while barium salts produce green flames.
Conclusion: The Importance of Understanding Group 2A Ion Formation
Understanding the ionic behavior of Group 2A elements is fundamental to comprehending their chemical reactivity and the properties of their compounds. The consistent formation of divalent cations, influenced by their electronic configuration and ionization energies, is the foundation for their wide range of applications across various industries and their essential roles in biological systems. Further research continues to unveil new aspects of their chemistry, promising continued innovation and utilization of these vital elements. The interplay of factors like ionic radius, charge density, and the resulting properties of their compounds makes this area of chemistry both fascinating and crucial for numerous scientific and technological advancements.
Latest Posts
Latest Posts
-
What Is The Mass Of One Atom Of Carbon 12
Apr 08, 2025
-
An Ion With A Negative Charge Is Called
Apr 08, 2025
-
What Is The Value Of Log 27 9
Apr 08, 2025
-
Is Air An Element Compound Homogeneous Or Heterogeneous
Apr 08, 2025
-
Osmosis Is A Type Of Active Transport
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about When Group 2a Elements Form Ions They . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.