What's The Mass Of A Proton
News Leon
Mar 20, 2025 · 7 min read
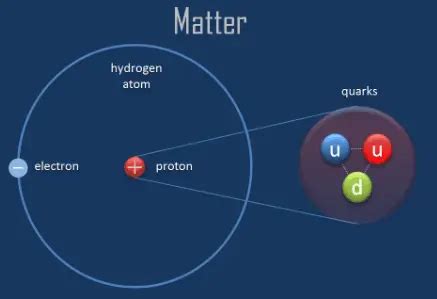
Table of Contents
What's the Mass of a Proton? A Deep Dive into Subatomic Physics
The seemingly simple question, "What's the mass of a proton?" opens a fascinating window into the world of subatomic particles and the fundamental forces that govern our universe. While a concise answer might seem straightforward, a deeper understanding requires exploring various measurement methods, units, and the inherent complexities of quantum mechanics. This article will delve into the mass of a proton, examining its different representations, the methods used to determine it, and its significance in physics and beyond.
Defining the Proton's Mass: A Matter of Units and Precision
The proton, a fundamental constituent of atomic nuclei, possesses a mass that is incredibly small by macroscopic standards. However, expressing this mass accurately requires careful consideration of units and the level of precision needed.
Mass in Kilograms (kg): The SI Standard
The International System of Units (SI) defines the kilogram (kg) as the base unit of mass. The mass of a proton in kilograms is approximately 1.6726219 × 10<sup>-27</sup> kg. This number represents an incredibly small fraction of a kilogram, highlighting the minuscule scale of the subatomic world. The precision to which this value is known is impressive, a testament to the advancements in experimental techniques.
Mass in Atomic Mass Units (amu or u): A More Convenient Scale
For many applications in physics and chemistry, using kilograms to express the mass of protons and other subatomic particles proves inconvenient. This is where the atomic mass unit (amu or u) comes in. One atomic mass unit is defined as one twelfth of the mass of a carbon-12 atom. This unit simplifies calculations significantly, making it easier to compare the masses of various isotopes and molecules. In atomic mass units, the proton's mass is approximately 1.007276 u. This value is remarkably close to 1, indicating that the proton's mass serves as a convenient relative standard for other atomic particles.
Mass-Energy Equivalence: E=mc²
Einstein's famous equation, E=mc², reveals the profound relationship between mass and energy. This equation demonstrates that mass and energy are fundamentally interchangeable. The proton's mass can also be expressed in terms of its energy equivalence. This conversion allows physicists to discuss particle masses in terms of energy units like electronvolts (eV) or mega-electronvolts (MeV). The proton's mass-energy equivalence is approximately 938.272 MeV/c². This expression emphasizes the immense energy contained within even a single proton.
Methods for Determining Proton Mass: A Journey Through Experimental Physics
Accurately measuring the mass of a proton presents significant experimental challenges. The minuscule size and extremely high speed of protons require sophisticated techniques. Several approaches have been employed to achieve this remarkable feat of precision.
Mass Spectrometry: Weighing Atoms and Ions
Mass spectrometry is a powerful technique used to determine the mass-to-charge ratio of ions. By accelerating ions through a magnetic field, scientists can separate them based on their mass-to-charge ratios. Careful calibration and analysis of the resulting spectra allow for the determination of the proton's mass. This technique relies on comparing the proton's mass to that of other known ions, providing a highly accurate relative measurement. The accuracy of mass spectrometry depends on the precision of the magnetic field, the detectors, and the calibration standards used.
Penning Traps: Precision Measurements in Electromagnetic Fields
Penning traps utilize a combination of electric and magnetic fields to confine charged particles. These traps enable the precise measurement of the cyclotron frequency of a trapped proton. This frequency is directly related to the proton's mass-to-charge ratio. By using highly stable and precisely controlled magnetic fields, Penning traps have been instrumental in determining the proton's mass with exceptional accuracy. The stability of the electromagnetic fields and the sophisticated detection systems are critical to the precision achieved in Penning trap experiments.
Combining Data from Multiple Experiments: Reducing Uncertainty
Determining the proton's mass with the highest accuracy relies on combining data from multiple independent experiments. Different methods each have their strengths and weaknesses, with various sources of uncertainty. By employing a statistical analysis that combines results from various techniques, scientists can reduce the overall uncertainty and obtain a more reliable estimate of the proton's mass. This process highlights the collaborative nature of scientific research and the power of combining complementary experimental approaches.
The Significance of Proton Mass: Implications Across Physics
The precise determination of the proton's mass has far-reaching implications for numerous areas of physics and beyond:
Understanding Fundamental Forces: The Standard Model
The proton's mass plays a crucial role in our understanding of the fundamental forces of nature as described by the Standard Model of particle physics. The mass of the proton, along with the masses of other elementary particles, is directly linked to the Higgs field. The interaction of particles with the Higgs field is responsible for giving them mass. Accurately measuring the proton's mass allows physicists to test and refine the Standard Model, searching for discrepancies that might hint at new physics beyond the established framework.
Nuclear Physics and Astrophysics: Stellar Nucleosynthesis
In nuclear physics, the proton's mass is critical in determining the properties of atomic nuclei. Understanding the mass of protons and neutrons allows physicists to model nuclear reactions, such as those that occur within stars. This is vital for understanding stellar nucleosynthesis, the process by which elements are created in stars. The proton's mass influences the binding energies of nuclei and the stability of various isotopes.
Chemistry and Materials Science: Molecular Properties
The proton's mass also affects the properties of molecules and materials. In chemical reactions, the mass of protons and other nuclei influences the vibrational frequencies of molecules and the strength of chemical bonds. In materials science, the mass of atoms plays a role in determining the physical and chemical properties of materials.
Particle Physics Experiments: Precision Measurements and New Physics
High-energy particle physics experiments require precise knowledge of the proton's mass. For example, in collider experiments, scientists use protons as projectiles to probe the structure of matter at the smallest scales. Accurate measurements of the proton's mass are necessary to accurately interpret the results of these experiments and search for new particles and phenomena.
Ongoing Research and Future Prospects: Refining Our Understanding
Despite the current high precision in determining the proton's mass, research continues to refine these measurements. Scientists are constantly seeking to improve the accuracy of experimental techniques and to reduce systematic uncertainties. Further advancements in technology and experimental design could lead to even more precise determinations of the proton's mass.
This ongoing pursuit of greater precision is vital for addressing outstanding questions in physics:
-
The Proton Radius Puzzle: Recent measurements of the proton's charge radius have revealed discrepancies between different experimental techniques. Resolving this puzzle could require further refinement of the proton's mass and a deeper understanding of the proton's internal structure.
-
Beyond the Standard Model: Any deviation from the predicted mass of the proton based on the Standard Model could indicate the existence of new physics beyond our current understanding. High-precision measurements of the proton's mass offer a crucial test of the Standard Model and a potential pathway to discovering new phenomena.
Conclusion: A Tiny Particle, A Vast Impact
The seemingly simple question of the proton's mass leads to a complex and fascinating journey into the heart of subatomic physics. The precision with which we can now measure this fundamental property is a testament to the remarkable advancements in experimental techniques. This understanding of the proton's mass is not merely an academic pursuit; it has profound implications for various fields of science and our understanding of the universe itself. The ongoing research and future advancements in measurement techniques promise to further refine our knowledge of this fundamental building block of matter, potentially uncovering new secrets of the cosmos along the way.
Latest Posts
Latest Posts
-
75 Percent Of What Number Is 15
Mar 21, 2025
-
Time Magazine Person Of The Century 1999
Mar 21, 2025
-
Ground State Electron Configuration For Titanium
Mar 21, 2025
-
Which Of The Following Is The Most Acidic
Mar 21, 2025
-
The Primary Function Of The Excretory System Is To
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What's The Mass Of A Proton . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
