What Protein Is The Most Important Buffer In Blood Plasma
News Leon
Mar 20, 2025 · 5 min read
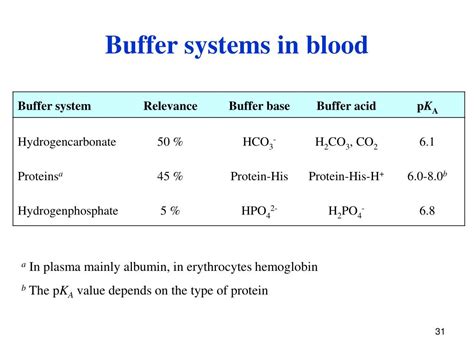
Table of Contents
What Protein is the Most Important Buffer in Blood Plasma?
Maintaining a stable pH within a narrow range is crucial for the proper functioning of biological systems. Deviations from this optimal pH can significantly impact enzyme activity, protein structure, and overall cellular health. In blood plasma, this critical pH regulation is achieved through a sophisticated buffering system, primarily involving proteins. While several proteins contribute, albumin stands out as the most important buffer in blood plasma. This article will delve deep into the role of albumin in maintaining blood pH, exploring its unique properties and comparing it to other contributing proteins.
The Importance of Blood Plasma pH
Blood plasma, the liquid component of blood, maintains a remarkably stable pH between 7.35 and 7.45. This tight regulation is essential because even slight shifts outside this range can lead to serious medical conditions like acidosis (pH below 7.35) or alkalosis (pH above 7.45). These conditions can disrupt cellular processes, affecting oxygen transport, enzyme function, and nerve impulse transmission. The consequences can range from mild discomfort to life-threatening complications.
The body employs several mechanisms to maintain blood pH homeostasis, with the buffering system playing a central role. Buffers are substances that resist changes in pH when acids or bases are added. They do this by either absorbing excess hydrogen ions (H+) when the pH drops or releasing hydrogen ions when the pH rises. This minimizes the impact of pH fluctuations, keeping the blood within the physiological range.
Albumin: The Dominant Blood Plasma Buffer
Among the various proteins in blood plasma, albumin emerges as the most significant buffer. Its abundance and unique properties make it exceptionally well-suited for this vital task. Several factors contribute to albumin's dominance as a blood buffer:
1. High Concentration:
Albumin is the most abundant protein in blood plasma, representing approximately 50-60% of total plasma protein. This high concentration directly translates to a greater buffering capacity. More albumin molecules mean a larger pool of available buffering sites to neutralize acids or bases.
2. Multiple Ionizable Groups:
Albumin's molecular structure boasts numerous ionizable groups, including carboxyl (-COOH) and amino (-NH2) groups. These groups can readily accept or donate protons (H+), depending on the surrounding pH. This versatility allows albumin to function efficiently across a range of pH values. When the pH decreases (becomes more acidic), the carboxyl groups can accept H+, while when the pH increases (becomes more alkaline), the amino groups can release H+. This dynamic equilibrium is key to its buffering effectiveness.
3. Amphoteric Nature:
Albumin is an amphoteric molecule, meaning it can act as both an acid and a base. This dual functionality enhances its buffering capabilities, allowing it to effectively neutralize both acids and bases. This is crucial because blood is exposed to a range of acidic and alkaline substances.
4. Binding Capacity:
Albumin doesn't just buffer through direct proton exchange. Its remarkable binding capacity for various substances, including many drugs and metabolites, indirectly impacts buffering. By binding to acidic or basic compounds, albumin can reduce their free concentration in the blood, thereby mitigating their influence on pH.
Other Contributing Plasma Proteins
While albumin is the primary buffer, other plasma proteins also contribute, albeit to a lesser extent:
Globulins:
Globulins represent a diverse group of proteins with various functions. Some globulins possess buffering capabilities, though their concentration is significantly lower than albumin's. Their contribution to overall blood buffering is less substantial compared to albumin.
Hemoglobin (in Red Blood Cells):
Though not strictly a plasma protein, hemoglobin plays a crucial role in buffering within red blood cells. Hemoglobin’s histidine residues act as significant buffers, particularly within the erythrocyte's environment. However, it is important to differentiate between intracellular buffering within red blood cells and the buffering capacity of plasma proteins.
Comparing Albumin's Buffering Capacity to Other Systems
The blood's buffering system isn't solely reliant on proteins. Other components contribute to maintaining pH homeostasis:
Bicarbonate Buffer System:
The bicarbonate buffer system, involving carbonic acid (H2CO3) and bicarbonate ions (HCO3-), is another crucial player in blood pH regulation. It is particularly efficient in response to respiratory changes in CO2 levels. However, the bicarbonate buffer system operates alongside the protein buffering system, complementing rather than replacing its function. They work synergistically to maintain pH stability.
Phosphate Buffer System:
The phosphate buffer system, comprised of dihydrogen phosphate (H2PO4-) and monohydrogen phosphate (HPO42-), contributes to buffering, especially in the renal system. Its capacity is lower compared to the bicarbonate and protein systems.
Clinical Significance of Albumin's Buffering Role
Hypoalbuminemia, a condition characterized by low albumin levels in the blood, can have significant implications for blood pH regulation. With reduced albumin, the buffering capacity of the plasma is compromised, increasing the susceptibility to acidosis or alkalosis. This can have serious consequences, particularly in patients with underlying conditions that affect protein synthesis or renal function.
Maintaining adequate albumin levels is therefore crucial for preserving blood pH homeostasis. Clinical monitoring of albumin levels is essential for assessing overall health and identifying potential risks associated with impaired pH regulation.
Conclusion
In summary, albumin reigns supreme as the most important buffer in blood plasma. Its high concentration, multiple ionizable groups, amphoteric nature, and binding capacity combine to create an exceptionally effective buffering system. While other proteins and non-protein components contribute, albumin’s dominance makes it the primary defense against significant fluctuations in blood pH, ensuring the proper functioning of the body's intricate biological processes. Maintaining adequate albumin levels is crucial for overall health and preventing complications associated with pH imbalances. Further research into albumin's complex structure and interaction with other buffering systems will continue to deepen our understanding of this vital physiological process. This robust buffering capacity underscores the importance of this protein in maintaining life's delicate chemical balance.
Latest Posts
Latest Posts
-
How Many Hours A Day Does A Cow Sleep
Mar 21, 2025
-
Which Of The Following Is Not A Product Of Photosynthesis
Mar 21, 2025
-
How Many Centimeters In A Picometer
Mar 21, 2025
-
Lecithin Is An Example Of A
Mar 21, 2025
-
Which Of The Following Is Equal To 5 1 3
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Protein Is The Most Important Buffer In Blood Plasma . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
