What Is The Molecular Mass Of H2o2
News Leon
May 05, 2025 · 5 min read
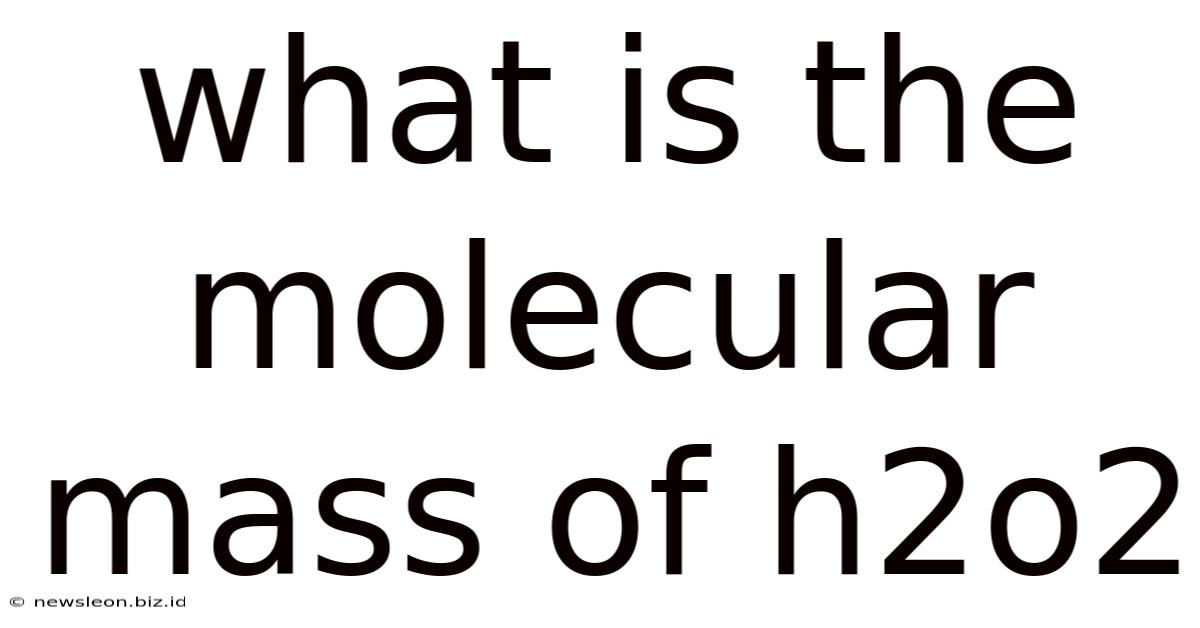
Table of Contents
What is the Molecular Mass of H₂O₂? A Deep Dive into Hydrogen Peroxide
Hydrogen peroxide (H₂O₂) is a common chemical compound with a wide range of applications, from everyday household cleaning to industrial processes. Understanding its properties, including its molecular mass, is crucial for various scientific and practical purposes. This article delves deep into the calculation and significance of the molecular mass of H₂O₂, exploring related concepts and applications.
Understanding Molecular Mass
Before calculating the molecular mass of H₂O₂, let's clarify the concept. Molecular mass, also known as molecular weight, refers to the mass of a molecule. It's calculated by summing the atomic masses of all the atoms present in the molecule. The unit of molecular mass is typically the atomic mass unit (amu) or Dalton (Da). It's important to differentiate molecular mass from molar mass. While molecular mass refers to the mass of a single molecule, molar mass refers to the mass of one mole (6.022 x 10²³ particles) of a substance, expressed in grams per mole (g/mol). Although numerically equivalent, their units differ significantly.
Calculating the Molecular Mass of H₂O₂
Hydrogen peroxide (H₂O₂) consists of two hydrogen atoms (H) and two oxygen atoms (O). To calculate its molecular mass, we need the atomic masses of hydrogen and oxygen. Standard atomic masses are typically obtained from the periodic table.
- Atomic mass of Hydrogen (H): Approximately 1.008 amu
- Atomic mass of Oxygen (O): Approximately 15.999 amu
Now, let's calculate the molecular mass of H₂O₂:
(2 x Atomic mass of H) + (2 x Atomic mass of Oxygen) = Molecular mass of H₂O₂
(2 x 1.008 amu) + (2 x 15.999 amu) = 34.014 amu
Therefore, the molecular mass of H₂O₂ is approximately 34.014 amu or Da. This value is crucial in various chemical calculations, including stoichiometry and concentration determination. Slight variations in the molecular mass might occur depending on the isotopic composition of the sample, but the value calculated above represents the standard molecular mass based on the average atomic masses.
Isotopic Variations and their Impact
The atomic masses used in the calculation above represent the average atomic masses of hydrogen and oxygen, considering the natural abundance of their isotopes. Hydrogen has two main isotopes: ¹H (protium) and ²H (deuterium). Oxygen has three main stable isotopes: ¹⁶O, ¹⁷O, and ¹⁸O. The presence of different isotopes can slightly alter the molecular mass of H₂O₂. For instance, a molecule of H₂O₂ containing deuterium instead of protium would have a higher molecular mass. However, for most practical purposes, the average molecular mass of 34.014 amu is sufficiently accurate.
Applications of Knowing the Molecular Mass of H₂O₂
Understanding the molecular mass of H₂O₂ is vital in many applications, including:
1. Stoichiometric Calculations:
In chemical reactions involving H₂O₂, knowing its molecular mass is essential for accurate stoichiometric calculations. This allows chemists to determine the precise amounts of reactants and products involved in a reaction. For example, if you're reacting H₂O₂ with another substance, the molecular mass helps determine the molar ratios and the amount of product formed.
2. Concentration Determination:
The molecular mass is crucial in determining the concentration of H₂O₂ solutions. Concentration is often expressed in molarity (moles per liter), which requires the molecular mass for conversion between mass and moles. This is critical in various applications, from analytical chemistry to industrial processes involving H₂O₂ solutions.
3. Spectroscopic Analysis:
Molecular mass plays a role in interpreting spectroscopic data. Techniques like mass spectrometry can directly measure the mass-to-charge ratio of molecules, which is related to the molecular mass. This helps identify and quantify H₂O₂ in complex mixtures.
4. Pharmaceutical and Medical Applications:
H₂O₂ finds applications in pharmaceuticals and medicine, such as disinfectants and wound care. Understanding its molecular mass aids in formulating appropriate concentrations and studying its interactions with biological systems.
5. Environmental Monitoring:
H₂O₂ is involved in various environmental processes. Knowing its molecular mass is helpful in environmental monitoring and assessing its impact on ecosystems. For instance, it's important to determine the concentration of H₂O₂ in water samples to evaluate water quality.
Beyond the Molecular Mass: Other Properties of H₂O₂
While molecular mass is a fundamental property, other properties of H₂O₂ are equally important:
- Chemical Formula: H₂O₂
- Chemical Name: Hydrogen Peroxide
- Appearance: Colorless liquid
- Odor: Slightly pungent
- Density: 1.11 g/cm³ (at 20°C)
- Boiling Point: 150.2 °C (decomposes)
- Melting Point: -0.43 °C
- Solubility in Water: Miscible
- Oxidizing Agent: A strong oxidizing agent, readily releasing oxygen.
- Reactivity: Reacts with various substances, often leading to decomposition or oxidation reactions.
- Toxicity: Corrosive and can cause skin and eye irritation.
Safety Precautions when Handling H₂O₂
Hydrogen peroxide, even in diluted forms, requires careful handling due to its reactive nature and potential hazards. Always follow safety protocols:
- Eye Protection: Wear safety goggles to prevent eye contact.
- Protective Clothing: Wear appropriate gloves and protective clothing to prevent skin contact.
- Ventilation: Ensure adequate ventilation to avoid inhaling fumes.
- Storage: Store H₂O₂ in a cool, dark place away from flammable materials.
- Disposal: Dispose of H₂O₂ solutions properly according to local regulations.
- First Aid: In case of contact with skin or eyes, rinse thoroughly with water and seek medical attention if needed.
Conclusion: The Importance of Molecular Mass in Understanding H₂O₂
The molecular mass of H₂O₂, approximately 34.014 amu, is a fundamental property that underpins many applications of this versatile chemical compound. This value is essential for precise stoichiometric calculations, concentration determination, and various analytical techniques. Understanding the molecular mass, combined with knowledge of other physical and chemical properties and safety precautions, allows for safe and effective use of hydrogen peroxide in various scientific, industrial, and domestic contexts. Remember that while the average molecular mass provides a sufficient estimate, isotopic variations can subtly influence the exact mass for highly sensitive applications. Always refer to reliable sources for accurate values and safety guidelines when working with H₂O₂.
Latest Posts
Related Post
Thank you for visiting our website which covers about What Is The Molecular Mass Of H2o2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.