What Is The Molar Mass Of Helium
News Leon
Mar 17, 2025 · 5 min read
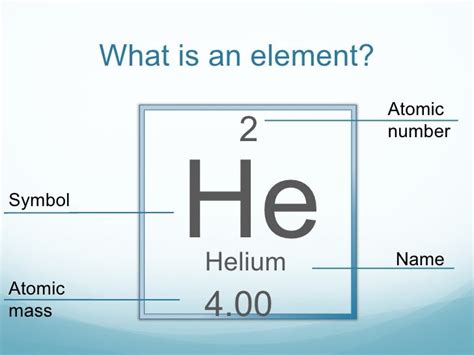
Table of Contents
What is the Molar Mass of Helium? A Deep Dive into Atomic Weight and Applications
Helium, the second element on the periodic table, is a fascinating and incredibly useful noble gas. Understanding its properties, particularly its molar mass, is crucial to appreciating its widespread applications in various fields, from cryogenics to medical imaging. This comprehensive guide delves into the concept of molar mass, explores the specific molar mass of helium, and examines its significance across diverse scientific and technological domains.
Understanding Molar Mass: The Foundation
Before we pinpoint the molar mass of helium, let's establish a solid understanding of the concept itself. Molar mass, often represented as M, is the mass of one mole of a substance. A mole, a fundamental unit in chemistry, is defined as the amount of a substance that contains as many elementary entities (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. This number, known as Avogadro's number, is approximately 6.022 x 10<sup>23</sup>.
In simpler terms, molar mass tells us the mass of a large collection of atoms or molecules, expressed in grams per mole (g/mol). It's a crucial link between the microscopic world of atoms and the macroscopic world of measurable quantities. It allows us to easily convert between the mass of a substance and the number of moles, which is essential for stoichiometric calculations and other chemical analyses.
Calculating Molar Mass: A Step-by-Step Guide
Calculating the molar mass of an element is straightforward. It's simply the atomic weight of the element expressed in grams per mole. The atomic weight, in turn, is the weighted average of the masses of all naturally occurring isotopes of that element, taking into account their relative abundances.
For instance, chlorine has two main isotopes: chlorine-35 and chlorine-37. Chlorine-35 is more abundant, making the weighted average atomic weight of chlorine slightly closer to 35 than 37. This weighted average is the value found on the periodic table and is used to calculate the molar mass.
The Molar Mass of Helium: A Precise Measurement
Helium, unlike chlorine, is predominantly composed of one isotope: helium-4. This makes the calculation of its molar mass particularly simple. The atomic weight of helium, as listed on the periodic table, is approximately 4.0026 atomic mass units (amu). Therefore, the molar mass of helium is approximately 4.0026 g/mol.
This value is incredibly precise and consistent across various sources due to helium's isotopic simplicity. The small variation you might find across different data sources is usually attributed to the level of precision in the measurement techniques used to determine the atomic weight.
Isotopes and their Influence: A Deeper Look
While helium-4 is the dominant isotope, trace amounts of helium-3 also exist in nature. Helium-3 has a slightly lower atomic mass than helium-4. However, its abundance is so small that its influence on the overall molar mass of helium is negligible, justifying the use of 4.0026 g/mol as a highly accurate approximation. The presence of helium-3 is noteworthy in certain scientific applications, particularly in nuclear physics and low-temperature research.
Applications of Helium and the Significance of its Molar Mass
The unique properties of helium, directly linked to its low atomic mass and inert nature, underpin its diverse applications in numerous fields. The precise knowledge of its molar mass is critical for accurate calculations in these applications.
1. Cryogenics and Superconductivity: Maintaining Extremely Low Temperatures
Helium's extremely low boiling point (-268.93 °C) makes it the cornerstone of cryogenics. Its molar mass plays a significant role in determining its physical properties at extremely low temperatures. The low molar mass contributes to its high rate of diffusion and low density, making it ideal for cooling superconducting magnets used in MRI machines, particle accelerators, and other advanced technologies. The precise molar mass is necessary for accurately calculating the amount of helium needed to achieve and maintain the desired cryogenic temperatures.
2. Medical Imaging: MRI and Other Diagnostic Techniques
Magnetic Resonance Imaging (MRI) relies heavily on superconducting magnets cooled by liquid helium. Accurate calculations of helium's molar mass are vital for determining the precise amount of liquid helium required to maintain these magnets at their operational temperatures. Errors in molar mass calculations can lead to malfunctioning equipment, compromising the accuracy and reliability of medical diagnoses.
3. Welding and Leak Detection: Taking Advantage of Helium's Inertness and Low Density
Helium's inertness and low density make it useful as a shielding gas in arc welding processes. It prevents oxidation and other undesirable reactions that could compromise the weld's quality. Furthermore, its low molar mass contributes to its high diffusion rate, making it ideal for detecting leaks in high-vacuum systems and other sensitive equipment. Precise calculations involving helium's molar mass ensure the efficient and effective use of the gas in these applications.
4. Aerospace and Balloons: Achieving Buoyancy and Lift
Helium's low density, a direct consequence of its low molar mass, makes it an ideal lifting gas for weather balloons, airships, and other aerospace applications. Knowing its precise molar mass allows engineers to accurately calculate the volume of helium needed to achieve the desired buoyancy and lift, critical for safe and effective operation.
5. Scientific Research: Diverse Applications Across Various Disciplines
Helium’s unique properties find application in countless areas of scientific research. Its inertness makes it an ideal carrier gas in chromatography, while its low molar mass allows for high resolution in mass spectrometry. The precision of helium's molar mass is paramount in these contexts, ensuring reliable experimental results and enabling scientists to draw meaningful conclusions.
Conclusion: The Importance of Precision and Understanding
The molar mass of helium, approximately 4.0026 g/mol, is not just a number; it's a fundamental property that underpins the element's widespread applications. Understanding its value and the principles behind its calculation is essential for anyone working in fields that utilize helium. From the intricacies of cryogenics to the life-saving applications of medical imaging, the accurate determination and utilization of helium's molar mass are indispensable for technological advancement and scientific progress. The seemingly simple number reflects the remarkable complexity and utility of this noble gas, and its importance in our modern world continues to grow.
Latest Posts
Latest Posts
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Helium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
