What Is The Molar Mass Of H3po4
News Leon
Mar 18, 2025 · 5 min read
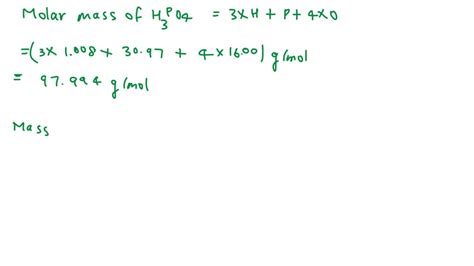
Table of Contents
What is the Molar Mass of H3PO4? A Comprehensive Guide
Determining the molar mass of a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. This article will delve into the process of calculating the molar mass of phosphoric acid (H₃PO₄), explaining the underlying principles and providing a detailed step-by-step guide. We'll also explore the significance of molar mass in different chemical contexts and address common misconceptions.
Understanding Molar Mass
Molar mass, often expressed in grams per mole (g/mol), represents the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as the amount of a substance containing Avogadro's number (approximately 6.022 x 10²³) of elementary entities (atoms, molecules, ions, etc.). Therefore, the molar mass provides a direct link between the mass of a substance and the number of particles it contains.
Why is Molar Mass Important?
Molar mass is an essential tool in many chemical calculations, including:
- Stoichiometry: Predicting the amounts of reactants and products in chemical reactions.
- Solution Chemistry: Determining concentrations of solutions (e.g., molarity).
- Titrations: Calculating the concentration of an unknown solution using a known solution.
- Gas Law Calculations: Relating the volume, pressure, and temperature of gases to their molar mass.
Calculating the Molar Mass of H3PO4 (Phosphoric Acid)
Phosphoric acid, H₃PO₄, is a common triprotic acid with numerous applications in various industries, from food production to fertilizer manufacturing. Calculating its molar mass involves adding the atomic masses of all the constituent atoms.
Step 1: Identify the Elements and their Atomic Masses
H₃PO₄ consists of three elements:
- Hydrogen (H): Atomic mass ≈ 1.008 g/mol
- Phosphorus (P): Atomic mass ≈ 30.974 g/mol
- Oxygen (O): Atomic mass ≈ 15.999 g/mol
Step 2: Determine the Number of Atoms of Each Element
The chemical formula H₃PO₄ indicates:
- 3 atoms of Hydrogen (H)
- 1 atom of Phosphorus (P)
- 4 atoms of Oxygen (O)
Step 3: Calculate the Total Mass Contribution of Each Element
Multiply the atomic mass of each element by the number of atoms present:
- Hydrogen: 3 atoms * 1.008 g/mol/atom = 3.024 g/mol
- Phosphorus: 1 atom * 30.974 g/mol/atom = 30.974 g/mol
- Oxygen: 4 atoms * 15.999 g/mol/atom = 63.996 g/mol
Step 4: Sum the Mass Contributions of All Elements
Add the mass contributions of each element to obtain the molar mass of H₃PO₄:
3.024 g/mol (H) + 30.974 g/mol (P) + 63.996 g/mol (O) = 97.994 g/mol
Therefore, the molar mass of H₃PO₄ is approximately 97.99 g/mol. The slight variation from the precise calculation might arise from using slightly different atomic mass values from different sources.
Common Misconceptions Regarding Molar Mass Calculations
Several common errors can occur when calculating molar mass:
- Incorrect Formula: Using the wrong chemical formula leads to inaccurate calculations. Double-checking the formula is crucial.
- Ignoring Subscripts: Forgetting to multiply the atomic mass by the number of atoms of each element is a frequent mistake.
- Inaccurate Atomic Masses: Using outdated or imprecise atomic masses can affect the accuracy of the results. It's always best to consult a reliable periodic table for the most up-to-date atomic masses.
- Unit Errors: Failing to include the correct units (g/mol) can lead to confusion and misinterpretations.
Applications of H3PO4 and the Importance of Molar Mass in its Use
The accurate determination of H₃PO₄'s molar mass is critical in its various applications:
1. Fertilizer Production:
Phosphoric acid is a vital component in the production of phosphate fertilizers, which are essential for plant growth. Precise calculations using its molar mass are necessary for formulating fertilizers with specific nutrient ratios. Knowing the molar mass allows manufacturers to accurately determine the amount of H₃PO₄ needed to achieve the desired concentration of phosphorus in the fertilizer. This ensures optimal plant nutrition without excessive application, contributing to sustainable agriculture.
2. Food and Beverage Industry:
H₃PO₄ acts as a food additive, primarily as an acidity regulator and flavor enhancer in various products, including soft drinks, processed foods, and jams. The molar mass is crucial for controlling the pH levels in these products and maintaining the desired taste and texture. Accurate molar mass calculations ensure consistent product quality and safety.
3. Chemical Synthesis:
H₃PO₄ plays a role as a catalyst or reagent in many chemical syntheses. Determining the precise amount of H₃PO₄ needed for a reaction is directly dependent on its molar mass, enabling chemists to control the reaction yield and product purity.
4. Rust Removal:
Phosphoric acid is a common ingredient in rust removers due to its ability to react with iron oxides, converting them into soluble phosphate salts. The molar mass is used to calculate the appropriate concentration of H₃PO₄ in the cleaning solution, ensuring effective rust removal without causing excessive damage to the underlying metal surface.
5. Dentistry:
In dentistry, phosphoric acid is used as an etchant to prepare tooth enamel for bonding procedures. Accurate molar mass calculations help determine the correct concentration of the acid to ensure proper etching without damaging the tooth structure, enabling successful and long-lasting dental restorations.
Conclusion
Calculating the molar mass of H₃PO₄, as demonstrated above, is a straightforward process that relies on understanding the chemical formula and the atomic masses of the constituent elements. This fundamental calculation is essential in various scientific disciplines and industrial applications, particularly concerning the production and use of phosphoric acid. Accuracy in molar mass calculations guarantees precision in stoichiometric calculations, solution preparation, and industrial processes, ultimately impacting product quality, safety, and efficiency. Understanding and correctly applying these calculations is vital for anyone working in chemistry or related fields.
Latest Posts
Latest Posts
-
Is Soil A Homogeneous Or Heterogeneous Mixture
Mar 18, 2025
-
What Does Isinstance Do In Python
Mar 18, 2025
-
What Is The Difference Between Political Parties And Interest Groups
Mar 18, 2025
-
Electromagnetic Radiation At Its Maximum Wavelength Is
Mar 18, 2025
-
Who Is The Writer Of Vande Mataram
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of H3po4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
