What Is The Molar Mass Of Acetic Acid
News Leon
Mar 18, 2025 · 5 min read
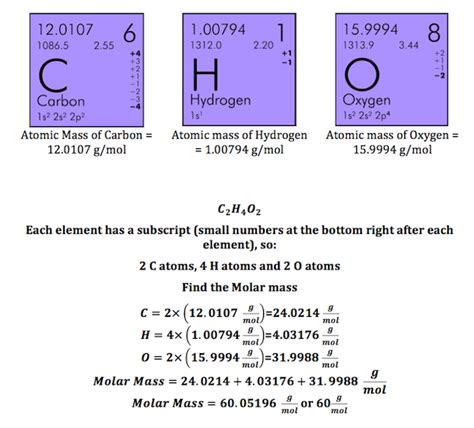
Table of Contents
What is the Molar Mass of Acetic Acid? A Deep Dive into Molecular Weight and its Applications
Acetic acid, a ubiquitous compound in our daily lives, plays a significant role in various industries and biological processes. Understanding its properties, particularly its molar mass, is crucial for numerous applications, from chemical reactions to industrial production. This comprehensive guide delves into the calculation, significance, and applications of the molar mass of acetic acid.
Understanding Molar Mass
Before we delve into the specifics of acetic acid, let's establish a clear understanding of molar mass. Molar mass is defined as the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10<sup>23</sup>) of particles (atoms, molecules, ions, etc.). The molar mass is typically expressed in grams per mole (g/mol).
The molar mass of an element is simply its atomic weight in grams per mole. For compounds, however, we must consider the molar mass of each constituent element and its number in the molecular formula.
Calculating the Molar Mass of Acetic Acid
Acetic acid, also known as ethanoic acid, has the chemical formula CH<sub>3</sub>COOH. To calculate its molar mass, we need to consider the molar mass of each element present:
- Carbon (C): 12.01 g/mol
- Hydrogen (H): 1.01 g/mol
- Oxygen (O): 16.00 g/mol
Now, let's break down the calculation:
- Carbon (C): 2 atoms x 12.01 g/mol = 24.02 g/mol
- Hydrogen (H): 4 atoms x 1.01 g/mol = 4.04 g/mol
- Oxygen (O): 2 atoms x 16.00 g/mol = 32.00 g/mol
Adding these values together, we get the molar mass of acetic acid:
24.02 g/mol + 4.04 g/mol + 32.00 g/mol = 60.06 g/mol
Therefore, the molar mass of acetic acid is approximately 60.06 g/mol. This value is essential for various stoichiometric calculations and other chemical analyses involving acetic acid.
The Significance of Molar Mass in Chemical Calculations
The molar mass of acetic acid is a cornerstone in numerous chemical calculations:
1. Stoichiometry
In stoichiometric calculations, the molar mass is crucial for converting between mass and moles. For instance, if you know the mass of acetic acid involved in a reaction, you can use its molar mass to determine the number of moles present. This allows you to accurately predict the amount of product formed or reactant consumed.
2. Concentration Calculations
Molar mass is essential for determining the concentration of acetic acid solutions. Molarity (mol/L), a common unit of concentration, is defined as the number of moles of solute (acetic acid) per liter of solution. Knowing the molar mass allows for precise conversion from mass to moles and consequently, accurate molarity calculations.
3. Titrations
In acid-base titrations, where acetic acid is often used, the molar mass is crucial for determining the concentration of the unknown solution. By using the molar mass, one can accurately calculate the number of moles of acetic acid that reacted with the titrant, leading to a precise determination of the unknown concentration.
4. Determining Empirical and Molecular Formulas
In analytical chemistry, the molar mass can be used in conjunction with elemental analysis data to determine the empirical and molecular formulas of unknown compounds. The molar mass provides a crucial piece of information needed to distinguish between possible formulas with the same empirical formula.
5. Gas Law Calculations
For gaseous acetic acid (though it's more commonly a liquid), the molar mass is crucial in calculations involving the ideal gas law (PV = nRT), where 'n' represents the number of moles. By knowing the molar mass, you can relate the volume, pressure, and temperature of the gas to its mass.
Applications of Acetic Acid and its Molar Mass
Acetic acid, with its diverse properties, finds applications across a wide range of fields:
1. Food Industry
Acetic acid is a key component in vinegar, a common condiment and preservative. Its molar mass is critical for regulating the concentration in food products to meet safety and flavor standards.
2. Pharmaceutical Industry
Acetic acid is used as a solvent, reagent, and preservative in pharmaceutical manufacturing. Precise molar mass measurements are essential to ensure accurate dosage and formulation.
3. Chemical Industry
Acetic acid serves as a crucial precursor in the production of various chemicals, including vinyl acetate monomer (VAM) used in the production of polyvinyl acetate (PVAc) adhesives and paints. Accurate molar mass calculations are vital for efficient chemical process control.
4. Textile Industry
Acetic acid is used in dyeing and finishing textiles. Its molar mass helps in determining the appropriate concentration for effective processing and color consistency.
5. Biomedical Applications
Acetic acid exhibits antimicrobial properties and is utilized as a disinfectant and antiseptic. Precise molar mass measurements facilitate the creation of effective and safe solutions.
6. Agricultural Applications
Acetic acid plays a role in controlling weeds and pests in agriculture. Knowing its molar mass helps in calculating the appropriate dilution for safe and effective application.
Beyond the Basics: Isotopes and Isotopic Abundance
The molar mass value of 60.06 g/mol we calculated is actually an average molar mass. This is because carbon, hydrogen, and oxygen each exist as various isotopes with slightly different masses. The average molar mass accounts for the natural abundance of each isotope. For very precise applications, it might be necessary to consider the isotopic composition of the acetic acid sample.
Conclusion
The molar mass of acetic acid, approximately 60.06 g/mol, is a fundamental property with far-reaching implications across multiple disciplines. Its accurate determination is crucial for a wide range of applications, from basic stoichiometric calculations to sophisticated industrial processes. Understanding its calculation and significance helps to appreciate the intricate relationship between molecular weight and the practical applications of this important compound. The implications extend beyond simple calculations, affecting quality control, safety regulations, and the efficiency of various industrial processes. Therefore, a thorough understanding of the molar mass of acetic acid is not just important for academic purposes but also for practical and industrial applications.
Latest Posts
Latest Posts
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Acetic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
