What Is The Mass Of An Electron In Amu
News Leon
Apr 06, 2025 · 6 min read
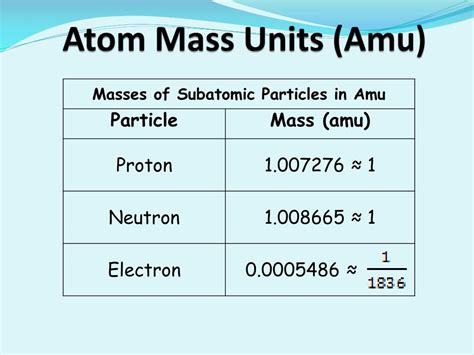
Table of Contents
What is the Mass of an Electron in amu? A Deep Dive into Subatomic Particles
The seemingly simple question, "What is the mass of an electron in amu?" opens a fascinating door into the world of quantum mechanics and the fundamental building blocks of matter. While the answer itself is straightforward, understanding its implications requires exploring the concepts of atomic mass units (amu), subatomic particles, and the complexities of measuring something as incredibly tiny as an electron.
Understanding Atomic Mass Units (amu)
Before we delve into the electron's mass, let's establish a firm grasp of the unit we're using: the atomic mass unit (amu), also known as the dalton (Da). The amu is a relative unit of mass used to express the mass of atoms and molecules. It's defined as one-twelfth the mass of a single unbound neutral atom of carbon-12 (¹²C). This means that a carbon-12 atom has a mass of exactly 12 amu.
This seemingly arbitrary choice is incredibly convenient for several reasons:
- Relative Scale: Using a reference atom like carbon-12 provides a consistent and universally understood scale for comparing the masses of other atoms and molecules.
- Practicality: The mass of carbon-12 is easily measurable, providing a reliable basis for the unit.
- Consistency: The definition ensures a unified standard for mass measurements in chemistry and physics.
Choosing ¹²C as the standard was based on its abundance and relative ease of measurement compared to other atoms.
The Electron: A Tiny Particle with a Big Impact
Electrons are fundamental subatomic particles carrying a single negative elementary charge. They orbit the nucleus of an atom, a tiny, dense region containing protons and neutrons. The electron's presence dictates the atom's chemical properties and how it interacts with other atoms to form molecules. Understanding its mass is crucial in understanding the overall mass of an atom and the behavior of matter itself.
Key Properties of Electrons:
- Charge: -1 elementary charge (approximately -1.602 x 10⁻¹⁹ Coulombs)
- Spin: 1/2 (a quantum property related to intrinsic angular momentum)
- Mass: This is the focus of our article, and we'll explore it in detail below.
- Wave-Particle Duality: Electrons exhibit both wave-like and particle-like properties, a central concept in quantum mechanics.
The Mass of an Electron in amu: The Answer and its Context
The mass of a single electron is approximately 5.48579909070 × 10⁻⁴ amu. This incredibly small number highlights the minuscule nature of subatomic particles compared to even the smallest atoms. It's important to note that this is an experimentally determined value, subject to minor refinements as measurement techniques improve.
This extremely small mass has significant consequences:
- Negligible contribution to atomic mass: The mass of electrons is so small compared to the masses of protons and neutrons that it is often neglected when calculating the atomic mass of an element. The atomic mass is predominantly determined by the number of protons and neutrons in the nucleus. This is why the mass number of an atom is usually a whole number, close to the atomic mass.
- Impact on atomic structure: Although negligible in terms of overall mass, the electron's charge and quantum properties have a profound impact on the atom's overall structure and chemical behavior. The arrangement of electrons in different energy levels determines an atom's reactivity and its ability to form chemical bonds.
- Influence on chemical reactions: The electron's role in chemical bonding is paramount. Atoms interact and react with each other by exchanging or sharing electrons. The mass of the electron itself plays a minor role in this interaction, but its charge and quantum behavior are absolutely critical.
Methods for Determining Electron Mass
Measuring the mass of an electron is a challenging task due to its incredibly small size and the principles of quantum mechanics. Several advanced experimental techniques are used to obtain precise measurements, including:
- Millikan's Oil Drop Experiment: This historic experiment, though not directly measuring electron mass, determined the elementary charge of the electron. Combining this with measurements of other properties allowed for the calculation of the electron's mass.
- Spectroscopy: Analyzing the spectra of atoms and ions provides information on the energy levels of electrons. By studying these energy levels, one can infer information about electron mass.
- Particle Accelerators: High-energy particle accelerators, such as those used in high-energy physics research, allow scientists to study the behavior of electrons at very high speeds. Measurements of their momentum and energy can be used to determine their mass.
- Advanced Measurement Techniques: Modern techniques, such as using high-precision magnetic fields and sophisticated detection methods, continue to refine the accuracy of electron mass measurements.
Comparing Electron Mass to Other Subatomic Particles
To further appreciate the smallness of the electron's mass, let's compare it to the masses of protons and neutrons:
- Proton Mass: Approximately 1.00727646688 amu
- Neutron Mass: Approximately 1.00866491588 amu
As you can see, the mass of a proton and neutron is roughly 1836 times greater than the mass of an electron. This substantial difference reinforces the dominant role of protons and neutrons in determining an atom's overall mass.
The Significance of Precise Electron Mass Measurement
The accurate measurement of the electron's mass is not just an academic exercise; it has significant implications in several fields:
- Fundamental Physics: Precise electron mass values are crucial in testing fundamental theories like the Standard Model of particle physics. Any deviation from predicted values could indicate new physics beyond the Standard Model.
- Atomic and Molecular Physics: Understanding the electron mass is essential for accurate calculations of atomic and molecular properties, including energy levels, bond lengths, and molecular vibrations. This has applications in various fields, including chemistry, materials science, and drug design.
- Astrophysics and Cosmology: The electron's mass is relevant in understanding the formation of stars and galaxies. Accurate mass measurements contribute to improved models of stellar evolution and the overall evolution of the universe.
- Technological Applications: The precise measurement of the electron's mass has implications for developing advanced technologies based on manipulating individual electrons and quantum phenomena, such as quantum computing and quantum sensing.
Beyond the Mass: The Electron's Quantum Nature
While the mass of an electron is a crucial property, it's vital to remember its wave-particle duality and other quantum properties. The electron isn't simply a tiny ball of matter; it behaves like a wave and its behavior is governed by the laws of quantum mechanics. Concepts like Heisenberg's uncertainty principle affect our ability to precisely know both the electron's position and momentum simultaneously.
The electron's wave nature is essential in understanding phenomena like electron diffraction and the formation of electron clouds around the nucleus, which dictate an atom's chemical behavior. The interaction of an electron's wave nature with electromagnetic fields leads to phenomena like the Zeeman effect, which is used in various spectroscopic techniques.
Conclusion: A Tiny Particle, a Vast Impact
The mass of an electron in amu, while a seemingly small number, represents a critical piece of the puzzle in understanding the fundamental building blocks of the universe. Its incredibly small mass compared to protons and neutrons, while often neglected in atomic mass calculations, doesn't diminish its importance. The electron's charge, quantum properties, and interactions with other particles fundamentally shape the world around us. The ongoing pursuit of ever-more precise measurements of its mass, along with a deep understanding of its quantum behavior, continues to drive advancements in fundamental physics, chemistry, and technology. The seemingly simple question, "What is the mass of an electron in amu?", thus reveals a much deeper story of scientific discovery and the intricacies of the subatomic world.
Latest Posts
Latest Posts
-
Patents Copyrights And Trademarks Are Examples Of
Apr 07, 2025
-
Area Of Circle With Radius Of 7
Apr 07, 2025
-
Example Of Law Of Conservation Of Charge
Apr 07, 2025
-
Camera Is Input Or Output Device
Apr 07, 2025
-
Molar Mass Of Alum Kal So4 2 12h2o
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Is The Mass Of An Electron In Amu . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
