What Is The Empirical Formula For C6h6
News Leon
Apr 07, 2025 · 7 min read
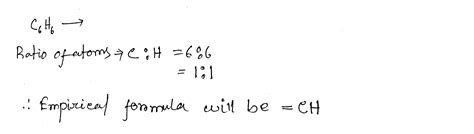
Table of Contents
What is the Empirical Formula for C₆H₆? Understanding Molecular and Empirical Formulas
The question, "What is the empirical formula for C₆H₆?" might seem deceptively simple at first glance. However, understanding the answer requires a firm grasp of the concepts of empirical and molecular formulas, their relationship, and how to derive one from the other. This article will delve into these concepts, explaining the difference between empirical and molecular formulas, how to calculate empirical formulas, and ultimately answer the question about the empirical formula for C₆H₆. We'll also explore the broader implications of this seemingly simple chemical formula.
Understanding Molecular and Empirical Formulas
Before we dive into the specifics of C₆H₆, let's clarify the definitions of molecular and empirical formulas.
Molecular Formula: This formula represents the actual number of atoms of each element present in a single molecule of a compound. For example, the molecular formula for glucose is C₆H₁₂O₆, indicating that one molecule of glucose contains 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
Empirical Formula: This formula represents the simplest whole-number ratio of atoms of each element in a compound. It's the most simplified version of the molecular formula. The empirical formula for glucose, C₆H₁₂O₆, would be CH₂O because the ratio of carbon to hydrogen to oxygen is 1:2:1. This means that for every one carbon atom, there are two hydrogen atoms and one oxygen atom. It doesn't tell us the actual number of atoms in a single molecule, just the ratio between them.
Determining Empirical Formulas: A Step-by-Step Guide
Calculating the empirical formula of a compound typically involves the following steps:
-
Determine the mass of each element present: This is often obtained through experimental methods like combustion analysis.
-
Convert the mass of each element to moles: Divide the mass of each element by its atomic weight (found on the periodic table).
-
Determine the mole ratio of each element: Divide the number of moles of each element by the smallest number of moles calculated in step 2. This will give you the ratio of atoms in the simplest whole-number form.
-
Write the empirical formula: Use the mole ratios as subscripts for each element in the chemical formula. If the ratios aren't whole numbers, you may need to multiply all the subscripts by a common factor to obtain whole numbers.
The Case of C₆H₆: Benzene
Now, let's address the question directly: What is the empirical formula for C₆H₆?
The molecular formula for benzene is C₆H₆. This means that one molecule of benzene contains 6 carbon atoms and 6 hydrogen atoms.
To find the empirical formula, we follow the steps outlined above:
-
Mass of Carbon and Hydrogen: While we don't have specific masses here, we know the ratio is 6:6.
-
Moles of Carbon and Hydrogen: The molar ratio reflects the atom ratio.
-
Mole Ratio: Dividing both by the smallest number of moles (which is 6 in this case), we get a ratio of 1:1.
-
Empirical Formula: Therefore, the empirical formula for C₆H₆ is CH.
The Significance of the Difference: Molecular vs. Empirical Formula in Benzene
The fact that benzene (C₆H₆) has a different molecular and empirical formula highlights a crucial distinction. Many compounds share the same empirical formula but have different molecular formulas. This means multiple compounds can have the same simplest ratio of atoms but differ in the total number of atoms in a single molecule. For example, acetylene (C₂H₂) and benzene (C₆H₆) both share the empirical formula CH, yet they are vastly different molecules with distinct properties.
The empirical formula provides a basic understanding of the elemental composition, but it doesn't fully describe the molecular structure or properties. The molecular formula, on the other hand, gives the complete picture of the molecule's composition. In the case of benzene, the molecular formula C₆H₆ reveals its unique cyclic structure and aromatic nature, which are crucial to understanding its chemical behavior.
Benzene: A Deeper Dive into its Properties and Importance
Benzene, with its molecular formula C₆H₆, is a significant organic compound in the chemical industry and scientific research. Its unique properties stem from its structure: a six-membered ring of carbon atoms with alternating single and double bonds (resonance structures). This leads to a delocalized electron cloud above and below the ring, making it exceptionally stable and reactive in specific ways.
Key properties of benzene include:
-
Aromatic Character: The delocalized electrons give benzene its aromatic character, making it relatively unreactive compared to other unsaturated hydrocarbons.
-
Planar Structure: The molecule is flat, due to the sp² hybridization of the carbon atoms.
-
Solubility: Relatively insoluble in water but soluble in nonpolar organic solvents.
-
Toxicity: Benzene is a known carcinogen and should be handled with extreme care.
Industrial applications of benzene are vast and include:
-
Production of Plastics: Benzene is a precursor to various plastics and polymers.
-
Synthesis of Dyes and Pharmaceuticals: It's used extensively in the synthesis of dyes, pharmaceuticals, and other chemicals.
-
Fuel Additive: In the past, it was used as a fuel additive, though this is less common now due to its toxicity.
-
Solvent: Although its use as a solvent is declining due to safety concerns, it still finds niche applications.
Beyond the Empirical Formula: Understanding Chemical Structures
While the empirical formula (CH) gives a simplified representation of the elemental composition of benzene, it fails to capture the crucial information about the molecule's cyclic structure and the delocalization of electrons. Understanding the molecular structure is paramount to understanding its chemical behavior and reactivity. Various techniques like X-ray crystallography and NMR spectroscopy are used to determine the molecular structures of organic compounds, providing a wealth of information beyond what the empirical formula alone can reveal.
Empirical Formula Calculation Practice Problems
Let's reinforce the concept with a few more examples:
Example 1: A compound is found to contain 75% carbon and 25% hydrogen by mass. Determine its empirical formula.
-
Assume 100g sample: This means we have 75g of carbon and 25g of hydrogen.
-
Moles: 75g C / 12.01 g/mol = 6.24 mol C; 25g H / 1.01 g/mol = 24.75 mol H
-
Mole Ratio: 6.24 mol C / 6.24 mol = 1; 24.75 mol H / 6.24 mol = 3.97 ≈ 4
-
Empirical Formula: The empirical formula is CH₄.
Example 2: A compound contains 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen. Determine its empirical formula.
-
Assume 100g sample: 40g C, 6.7g H, 53.3g O
-
Moles: 40g C / 12.01 g/mol = 3.33 mol C; 6.7g H / 1.01 g/mol = 6.63 mol H; 53.3g O / 16.00 g/mol = 3.33 mol O
-
Mole Ratio: 3.33 mol C / 3.33 mol = 1; 6.63 mol H / 3.33 mol = 2; 3.33 mol O / 3.33 mol = 1
-
Empirical Formula: The empirical formula is CH₂O.
These examples demonstrate the process of deriving empirical formulas. Remember, the empirical formula is the simplest whole-number ratio of atoms, and it may or may not be the same as the molecular formula.
Conclusion: The Importance of Context and Deeper Analysis
While the empirical formula for C₆H₆ is CH, it is crucial to understand that this simplified representation doesn't convey the complete picture of the molecule's structure and properties. The molecular formula C₆H₆ and the understanding of its unique aromatic structure are essential for a complete comprehension of benzene's behavior and importance in chemistry and industry. This emphasizes the significance of moving beyond simple empirical formulas to explore the more nuanced and complex world of molecular structures and their implications. Always consider the context and strive for a comprehensive understanding, moving beyond the basic to appreciate the full complexity of chemical compounds.
Latest Posts
Latest Posts
-
24 As A Product Of Prime Factors
Apr 10, 2025
-
What Is The Value Of 4 In 475
Apr 10, 2025
-
Words That Start With The Same Letter Are Called
Apr 10, 2025
-
The Type Of Hydrocarbon That Is Used As Lubricant
Apr 10, 2025
-
Does Current Flow From High Potential To Low Potential
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about What Is The Empirical Formula For C6h6 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.