What Is The Conjugate Base Of Hco3
News Leon
Mar 18, 2025 · 6 min read
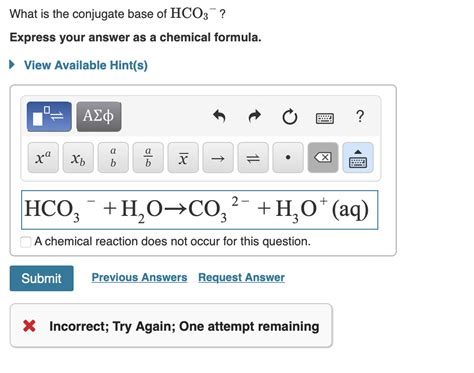
Table of Contents
What is the Conjugate Base of HCO₃⁻? Understanding Bicarbonate and its Acid-Base Chemistry
The bicarbonate ion, HCO₃⁻, plays a crucial role in numerous biological and chemical processes. Its ability to act as both an acid and a base stems from its amphoteric nature, meaning it can donate or accept a proton (H⁺). Understanding its conjugate base is key to grasping its behavior in various chemical environments. This comprehensive article delves deep into the concept of conjugate bases, focusing specifically on HCO₃⁻ and its significance in chemistry and biology.
Understanding Conjugate Acid-Base Pairs
Before diving into the specifics of HCO₃⁻, let's establish a fundamental understanding of conjugate acid-base pairs. According to the Brønsted-Lowry theory of acids and bases, an acid is a proton donor, and a base is a proton acceptor. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These two species are related by the difference of a single proton.
In simpler terms: Imagine a seesaw. The acid is on one side, and the base is on the other. The proton (H⁺) is the fulcrum. When the acid donates the proton, the seesaw tips, and the conjugate base is what remains on that side.
Identifying the Conjugate Base of HCO₃⁻
The bicarbonate ion, HCO₃⁻, can act as both an acid and a base. Let's examine its behavior in each scenario to identify its conjugate base:
HCO₃⁻ acting as an acid:
When HCO₃⁻ acts as an acid, it donates a proton (H⁺) to a base. The chemical equation representing this reaction is:
HCO₃⁻(aq) + H₂O(l) ⇌ CO₃²⁻(aq) + H₃O⁺(aq)
In this reaction:
- HCO₃⁻ is the acid (proton donor).
- H₂O is the base (proton acceptor).
- CO₃²⁻ is the conjugate base of HCO₃⁻ (what remains after HCO₃⁻ donates a proton).
- H₃O⁺ is the conjugate acid of H₂O (what is formed when H₂O accepts a proton).
Therefore, the conjugate base of HCO₃⁻ is carbonate ion (CO₃²⁻).
HCO₃⁻ acting as a base:
When HCO₃⁻ acts as a base, it accepts a proton (H⁺) from an acid. The chemical equation for this reaction is:
HCO₃⁻(aq) + H⁺(aq) ⇌ H₂CO₃(aq)
In this reaction:
- HCO₃⁻ is the base (proton acceptor).
- H⁺ is the acid (proton donor).
- H₂CO₃ is the conjugate acid of HCO₃⁻ (what is formed when HCO₃⁻ accepts a proton).
In this case, we're not directly looking for a conjugate base, but it's important to see the dual nature of HCO₃⁻.
The Significance of the Carbonate Ion (CO₃²⁻)
The carbonate ion (CO₃²⁻), the conjugate base of HCO₃⁻, is a crucial species in various chemical and biological systems:
1. Buffering Systems:
The HCO₃⁻/CO₃²⁻ buffer system is vital in maintaining the pH of blood and other biological fluids. This buffer system resists changes in pH by reacting with both acids and bases. When an acid is added, CO₃²⁻ reacts to neutralize it, and when a base is added, HCO₃⁻ reacts to neutralize it. This system's effectiveness is closely tied to the equilibrium between HCO₃⁻ and CO₃²⁻.
2. Carbonate Minerals:
Many important minerals are carbonates, including limestone (CaCO₃) and dolomite (CaMg(CO₃)₂). These minerals are formed through geological processes involving the precipitation of carbonate ions from aqueous solutions. Understanding the equilibrium between HCO₃⁻ and CO₃²⁻ is essential for understanding the formation and dissolution of these minerals.
3. Ocean Acidification:
The increase in atmospheric CO₂ leads to increased dissolution of CO₂ in the oceans, forming carbonic acid (H₂CO₃). This acid then dissociates to form HCO₃⁻ and H⁺ ions, ultimately increasing the acidity of the oceans. This process impacts marine life and ecosystems significantly. The equilibrium between HCO₃⁻ and CO₃²⁻ is crucial in determining the availability of carbonate ions for marine organisms like corals and shellfish to build their shells and skeletons. Lower concentrations of CO₃²⁻ due to increased acidity hamper shell formation and can lead to shell dissolution.
4. Industrial Applications:
Carbonate ions are used in various industrial applications, including:
- Cement production: Limestone (CaCO₃) is a key ingredient in cement production.
- Water softening: Carbonates are used to remove hardness ions (Ca²⁺ and Mg²⁺) from water.
- Chemical synthesis: Carbonates are used as reactants and catalysts in various chemical syntheses.
Further Exploring the Amphoteric Nature of HCO₃⁻
The amphoteric nature of HCO₃⁻ deserves further emphasis. It can act as both an acid and a base depending on the pH of the solution and the other species present.
In acidic solutions (low pH), HCO₃⁻ acts as a base, accepting a proton to form H₂CO₃.
In basic solutions (high pH), HCO₃⁻ acts as an acid, donating a proton to form CO₃²⁻.
This dual behavior is what makes it a crucial component in buffer systems, able to resist pH changes effectively.
Practical Applications and Real-World Examples
Let's explore some real-world scenarios where understanding the conjugate base of HCO₃⁻ is crucial:
-
Blood pH regulation: The bicarbonate buffer system in blood maintains a remarkably constant pH (approximately 7.4). Disruptions to this system, such as metabolic acidosis or alkalosis, can have severe health consequences. The equilibrium between HCO₃⁻ and CO₃²⁻ plays a central role in the body's ability to compensate for these pH imbalances.
-
Ocean acidification impact on marine ecosystems: As mentioned earlier, the increased concentration of CO₂ in the oceans lowers the pH and affects the availability of CO₃²⁻. This negatively impacts shell-forming organisms, leading to decreased shell growth and even dissolution in severe cases. This highlights the far-reaching environmental consequences of understanding this acid-base equilibrium.
-
Geological carbon sequestration: Scientists are exploring methods to capture CO₂ from industrial sources and store it safely underground. Understanding the interaction of CO₂ with carbonate rocks is crucial for evaluating the effectiveness and safety of such sequestration techniques. This involves a complex understanding of the equilibrium between CO₂, H₂CO₃, HCO₃⁻, and CO₃²⁻.
Conclusion: The Importance of Understanding Conjugate Bases
The conjugate base of HCO₃⁻, the carbonate ion (CO₃²⁻), plays a critical role in a wide range of chemical and biological processes. From regulating blood pH to impacting marine ecosystems and industrial applications, its significance cannot be overstated. Understanding its relationship with HCO₃⁻ and the equilibrium between them is essential for comprehending numerous natural phenomena and technological applications. This knowledge is crucial in fields ranging from medicine and environmental science to geology and materials science. Further research and exploration of this acid-base chemistry continue to unveil new insights into the complexities of natural and engineered systems. By understanding the conjugate base of HCO₃⁻ and its interactions, we gain a deeper understanding of the world around us.
Latest Posts
Latest Posts
-
Is Soil A Homogeneous Or Heterogeneous Mixture
Mar 18, 2025
-
What Does Isinstance Do In Python
Mar 18, 2025
-
What Is The Difference Between Political Parties And Interest Groups
Mar 18, 2025
-
Electromagnetic Radiation At Its Maximum Wavelength Is
Mar 18, 2025
-
Who Is The Writer Of Vande Mataram
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Base Of Hco3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
