What Is The Boiling Point In Kelvin
News Leon
Apr 06, 2025 · 6 min read
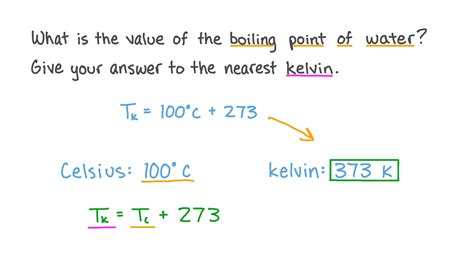
Table of Contents
What is the Boiling Point in Kelvin? Understanding Temperature and Phase Transitions
The boiling point, a fundamental concept in chemistry and physics, signifies the temperature at which a liquid transitions into a gaseous phase. While we often express boiling points in degrees Celsius or Fahrenheit, understanding the boiling point in Kelvin provides a deeper insight into the underlying thermodynamic principles governing this phase transition. This article will delve into the definition of boiling point in Kelvin, exploring the relationship between Kelvin, Celsius, and Fahrenheit, and examining factors influencing boiling points. We'll also explore the implications of boiling point in various applications and its significance in scientific research.
Defining Boiling Point in Kelvin
The boiling point in Kelvin, like in any other temperature scale, represents the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. At this point, bubbles of vapor form within the liquid and rise to the surface, leading to the characteristic vigorous bubbling associated with boiling. However, unlike Celsius or Fahrenheit which are arbitrary scales based on the freezing and boiling points of water, the Kelvin scale is an absolute temperature scale. This means zero Kelvin (0 K) represents absolute zero, the theoretically lowest possible temperature, where all molecular motion ceases.
Why is the Kelvin scale crucial for understanding boiling point? Because it's an absolute scale, changes in Kelvin directly reflect changes in the kinetic energy of molecules. A higher Kelvin temperature means molecules possess more kinetic energy, making it easier for them to overcome intermolecular forces and transition to the gaseous phase. This direct relationship makes Kelvin the preferred scale for scientific calculations involving temperature and phase transitions.
The Relationship Between Kelvin, Celsius, and Fahrenheit
To convert between Kelvin (K), Celsius (°C), and Fahrenheit (°F), you use the following formulas:
- K to °C: °C = K - 273.15
- °C to K: K = °C + 273.15
- °C to °F: °F = (°C × 9/5) + 32
- °F to °C: °C = (°F - 32) × 5/9
Understanding these conversions is essential for interpreting boiling point data presented in different units. For instance, the boiling point of water at standard atmospheric pressure is 100°C, which is equivalent to 373.15 K.
Factors Affecting Boiling Point in Kelvin
Several factors influence the boiling point of a substance, all of which ultimately affect the kinetic energy of the molecules and their ability to overcome intermolecular forces:
1. Intermolecular Forces:
Stronger intermolecular forces, such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces, require more energy to overcome. Substances with stronger intermolecular forces have higher boiling points in Kelvin. For example, water (H₂O), with its strong hydrogen bonding, has a significantly higher boiling point than methane (CH₄), which only exhibits weak London dispersion forces.
2. Molecular Weight:
Larger molecules generally have higher boiling points. This is because heavier molecules possess more electrons, leading to stronger London dispersion forces. The increased mass also contributes to lower kinetic energy at a given temperature, making it harder for the molecules to escape the liquid phase.
3. External Pressure:
The boiling point is directly affected by external pressure. At higher pressures, a higher temperature is needed to overcome the increased external force and achieve the vapor pressure required for boiling. Conversely, at lower pressures, the boiling point decreases. This principle is exploited in techniques like vacuum distillation, where reducing pressure lowers the boiling point of a substance, allowing for its purification at lower temperatures.
4. Impurities:
The presence of impurities in a liquid typically elevates its boiling point. These impurities disrupt the regular structure of the liquid, making it more difficult for molecules to escape into the gaseous phase. This phenomenon is known as boiling point elevation and is a colligative property, meaning it depends on the concentration of solute particles rather than their identity.
Applications of Boiling Point in Kelvin
The boiling point, expressed in Kelvin, plays a crucial role in various scientific, industrial, and everyday applications:
1. Chemical Processes:
Boiling points are essential in numerous chemical processes, including distillation, evaporation, and reflux. Distillation relies on the difference in boiling points of components in a mixture to separate them. Knowing the boiling points in Kelvin allows for precise control of temperature during these processes, ensuring efficient separation and preventing degradation of the desired compounds.
2. Material Science:
In material science, boiling point data is used to select appropriate solvents and processing conditions. Understanding the boiling point helps in designing materials with desired properties and predicting their behavior under different conditions. The boiling points of various solvents are critical in choosing the right solvent for a specific application.
3. Food Science:
Boiling points are fundamental in cooking and food processing. The boiling point of water, 373.15 K, is the basis for many cooking techniques like boiling, steaming, and pressure cooking. Understanding how pressure affects the boiling point is important in techniques like pressure cooking, which allows for faster cooking times at higher temperatures.
4. Meteorology and Climatology:
Boiling point is indirectly related to atmospheric pressure and temperature. Changes in atmospheric pressure and temperature affect the boiling point of water, influencing weather patterns and climate. Meteorologists utilize this knowledge to predict weather phenomena and understand climate change.
Boiling Point and Scientific Research
The boiling point in Kelvin serves as a crucial parameter in numerous scientific investigations:
1. Thermodynamic Studies:
Boiling point data are used to determine thermodynamic properties of substances, such as enthalpy of vaporization (the heat required to vaporize a liquid) and entropy of vaporization (the change in disorder during vaporization). These thermodynamic parameters offer vital information about the intermolecular forces and molecular structure of substances.
2. Phase Diagrams:
Boiling points are key components of phase diagrams, which graphically represent the phases of a substance as a function of temperature and pressure. Understanding the boiling point at different pressures is critical for predicting the behavior of a substance under various conditions.
3. Chemical Kinetics:
Boiling point can influence the rate of chemical reactions. The temperature affects the kinetic energy of reactants, influencing the reaction rate. Studying boiling points helps to control reaction conditions and optimize reaction yields.
4. Analytical Chemistry:
Boiling point is a physical property commonly used for identifying and characterizing unknown compounds. The boiling point, along with other properties, assists in identifying compounds through analytical techniques like gas chromatography and mass spectrometry.
Conclusion
The boiling point in Kelvin is not just a numerical value; it's a key indicator of a substance's molecular properties and its behavior under different conditions. The absolute nature of the Kelvin scale provides a more precise and fundamental understanding of the thermodynamic principles governing phase transitions. By understanding the factors influencing boiling points and their applications across diverse fields, we gain a deeper appreciation of this fundamental concept and its importance in scientific research, industrial processes, and everyday life. Further research continues to refine our understanding of boiling points and their implications, contributing to advancements in various scientific and technological domains.
Latest Posts
Latest Posts
-
What Happens When Two Forces Act In The Same Direction
Apr 07, 2025
-
What Structures Are Present Only In Animal Cells
Apr 07, 2025
-
What Are Two Parts Of Solution
Apr 07, 2025
-
Convert Hex String To Int Python
Apr 07, 2025
-
Which Of The Following Is Considered An Asset
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Is The Boiling Point In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
