What Happens When Nitrogen Fills Its Valence Shell
News Leon
Mar 21, 2025 · 6 min read
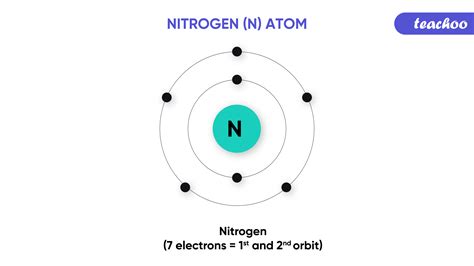
Table of Contents
What Happens When Nitrogen Fills Its Valence Shell: Exploring the Chemistry of Nitrogen Compounds
Nitrogen, a ubiquitous element crucial to life as we know it, exhibits fascinating chemical behavior primarily dictated by its electron configuration. Understanding what happens when nitrogen fills its valence shell is key to comprehending the vast array of nitrogen compounds and their diverse properties. This exploration delves into the fundamental principles, examining the impact on stability, reactivity, and the formation of various molecular structures.
Nitrogen's Electron Configuration: The Foundation of Reactivity
Nitrogen's atomic number is 7, meaning it possesses seven electrons. Its electron configuration is 1s²2s²2p³. This configuration reveals that the first energy level (n=1) is completely filled with two electrons, while the second energy level (n=2) has five electrons. Crucially, the outermost shell, or valence shell, contains five electrons – two in the 2s orbital and three in the 2p orbitals. This incomplete valence shell is the driving force behind nitrogen's reactivity. To achieve stability, nitrogen atoms strive to attain a full valence shell, mimicking the stable electron configuration of noble gases like neon (1s²2s²2p⁶).
The Octet Rule and Nitrogen's Pursuit of Stability
The octet rule, a cornerstone of chemical bonding theory, states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight electrons in their valence shell. For nitrogen, this translates to acquiring three more electrons. This can be accomplished through various mechanisms, notably:
- Covalent Bonding: Nitrogen readily forms covalent bonds by sharing electrons with other atoms. This is the most common way nitrogen achieves a filled valence shell. The shared electrons are counted towards the octet of each participating atom.
- Ionic Bonding (Less Common): While less prevalent than covalent bonding, nitrogen can form ionic bonds under specific conditions, typically involving highly electronegative atoms. In such cases, nitrogen may gain three electrons to form the nitride anion (N³⁻).
Consequences of a Filled Valence Shell: Stability and Molecular Structures
Once nitrogen achieves a filled valence shell, profound changes in its chemical behavior occur:
- Increased Stability: The primary consequence of a filled valence shell is a significant increase in stability. The atom's electronic configuration becomes much less reactive, reducing its tendency to participate in further chemical reactions. This stability is a crucial factor in the existence of numerous nitrogen-containing molecules.
- Diverse Molecular Structures: The way nitrogen atoms achieve a filled valence shell directly influences the shapes and properties of the resulting molecules. The number of covalent bonds formed, the presence of lone pairs of electrons, and the electronegativity differences between nitrogen and the bonded atoms all play a role in dictating molecular geometry and reactivity.
Examples of Nitrogen Compounds with Filled Valence Shells
The implications of nitrogen filling its valence shell are best illustrated through several examples:
1. Ammonia (NH₃)
Ammonia, a ubiquitous compound with the formula NH₃, exemplifies nitrogen achieving a filled valence shell through covalent bonding. Nitrogen shares three electrons, one with each of the three hydrogen atoms, forming three single covalent bonds. This arrangement provides nitrogen with eight valence electrons (two from the 2s orbital and six from the three shared pairs), fulfilling the octet rule and conferring relative stability. The geometry of ammonia is trigonal pyramidal due to the presence of one lone pair of electrons on the nitrogen atom.
2. Nitric Acid (HNO₃)
Nitric acid, a powerful oxidizing agent, features a nitrogen atom surrounded by several atoms. Nitrogen forms three single covalent bonds and a coordinate covalent bond, satisfying the octet rule. The molecule's structure is more complex, exhibiting resonance structures to reflect the delocalization of electrons. The presence of highly electronegative oxygen atoms significantly impacts the reactivity of the molecule.
3. Nitrogen Gas (N₂)
Nitrogen gas, N₂, represents perhaps the most important manifestation of nitrogen's tendency towards stability. In this diatomic molecule, two nitrogen atoms share three pairs of electrons, forming a triple bond. Each nitrogen atom contributes three electrons to the bond, resulting in a full octet for each. This triple bond is exceptionally strong, accounting for nitrogen's relatively low reactivity under standard conditions. This inertness of N₂ is crucial for its role in the atmosphere.
4. Ammonium Ion (NH₄⁺)
The ammonium ion (NH₄⁺) is a cation formed when ammonia accepts a proton (H⁺). In this case, nitrogen forms four covalent bonds, one with each of the four hydrogen atoms. This results in a filled valence shell with eight electrons, making it a stable cation. The geometry is tetrahedral.
Beyond the Octet Rule: Exceptions and Complexities
While the octet rule serves as a valuable guideline, it is not without exceptions, particularly when dealing with elements beyond the second row of the periodic table. Nitrogen, being in the second row, largely adheres to the octet rule. However, certain conditions may lead to deviations:
Hypervalent Compounds (Rare for Nitrogen)
Hypervalent compounds are molecules where the central atom possesses more than eight valence electrons. These are rare for nitrogen, due to its relatively small size and the higher energy levels required to accommodate additional electrons beyond the octet.
The Significance of Nitrogen and Its Compounds in Various Fields
The properties of nitrogen compounds, stemming from nitrogen’s ability to achieve a filled valence shell, have far-reaching implications across numerous fields:
1. Agriculture: Fertilizers
Ammonia and its derivatives are the foundation of nitrogen-based fertilizers, crucial for crop production. These fertilizers provide essential nitrogen for plant growth, boosting yields and ensuring food security.
2. Industry: Explosives and Propellants
Certain nitrogen compounds, like nitrates and nitroglycerin, are used in explosives and propellants due to their high energy content. The decomposition of these compounds releases a significant amount of energy, leading to their explosive nature.
3. Medicine: Pharmaceuticals and Anesthetics
Many pharmaceuticals and anesthetics contain nitrogen atoms, showcasing the importance of nitrogen in bioactive molecules.
4. Materials Science: Polymers and Composites
Nitrogen is incorporated into various polymers and composites to enhance their properties. These properties can include strength, durability, and thermal stability.
Conclusion: The Versatile Chemistry of Nitrogen
Understanding what happens when nitrogen fills its valence shell is pivotal to comprehending its extensive role in chemistry and countless applications. The attainment of a stable octet drives nitrogen's reactivity and shapes the structures and properties of its countless compounds. From the life-sustaining role of ammonia in agriculture to the energy-rich applications of explosives, the consequences of a filled nitrogen valence shell profoundly impact various aspects of modern life. Further research and development into nitrogen chemistry continue to expand our understanding and unlock new possibilities in various fields. The seemingly simple act of nitrogen achieving a full valence shell has far-reaching consequences, making it a key element to continue studying and appreciating.
Latest Posts
Latest Posts
-
Centrioles Move To Opposite Ends Of The Cell
Mar 28, 2025
-
What Are The Side Of The Dna Ladder Made Of
Mar 28, 2025
-
Which Of The Following Is Not Used As An Antiseptic
Mar 28, 2025
-
A Transverse Pulse Generated At The Bottom
Mar 28, 2025
-
How Many Moles Are In 9 8 G Of Calcium
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Happens When Nitrogen Fills Its Valence Shell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
