What Binds To The Exposed Cross Bridges On Actin
News Leon
Apr 04, 2025 · 5 min read
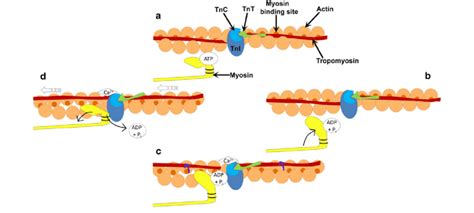
Table of Contents
What Binds to the Exposed Cross-Bridges on Actin? The Complex Dance of Muscle Contraction
Muscle contraction, a fundamental process in movement and life itself, hinges on a precisely orchestrated interaction between actin and myosin filaments. This intricate dance relies on the binding of myosin cross-bridges to specific sites on the actin filament. Understanding this binding mechanism is crucial to grasping the mechanics of muscle function, its regulation, and the implications of disruptions in this process leading to various muscle disorders. This article will delve deep into the intricacies of what binds to the exposed cross-bridges on actin, exploring the molecular players, regulatory mechanisms, and the broader implications for muscle physiology.
The Actin Filament: A Dynamic Structure
Before diving into the binding process, it's essential to understand the structure of the actin filament itself. Actin, a globular protein (G-actin), polymerizes to form long, helical filaments (F-actin). These filaments exhibit distinct features crucial for myosin binding. Most importantly, F-actin possesses binding sites, specifically within the cleft of each G-actin monomer, that are masked in the resting state. These masked binding sites are the key to regulated muscle contraction.
Myosin: The Molecular Motor
Myosin, another crucial protein in muscle contraction, is a motor protein responsible for generating the force needed for muscle shortening. Myosin molecules are composed of two heavy chains and several light chains. The heavy chains form a long tail region and a globular head region, termed the myosin head or cross-bridge. The myosin head contains the ATPase activity essential for generating the energy required for muscle contraction and contains the binding site for actin.
The Binding Event: Unveiling the Exposed Sites
The binding of myosin cross-bridges to actin is not a passive event but a highly regulated and dynamic process. The key to this regulation lies in the presence of tropomyosin and troponin complex on the actin filament.
Tropomyosin: The Steric Blocker
Tropomyosin, a long fibrous protein, lies in the groove of the actin filament, effectively blocking the myosin-binding sites on actin in the resting state. This steric hindrance prevents spontaneous myosin binding and uncontrolled muscle contraction.
Troponin: The Calcium Sensor
Troponin, a complex of three proteins (troponin I, troponin T, and troponin C), plays a pivotal role in regulating the position of tropomyosin. Crucially, troponin C binds calcium ions (Ca²⁺). This calcium binding triggers a conformational change in the troponin complex, which, in turn, shifts tropomyosin, uncovering the myosin-binding sites on actin.
The Cross-Bridge Cycle: A Step-by-Step Analysis
Once the myosin-binding sites are exposed, the cross-bridge cycle can begin. This cycle consists of several distinct steps:
-
Attachment: The myosin head, carrying ADP and inorganic phosphate (Pi), binds to the exposed myosin-binding site on actin. This binding event initiates the power stroke.
-
Power Stroke: Following the attachment, the myosin head undergoes a conformational change, pivoting and pulling the actin filament towards the center of the sarcomere. This conformational change releases the ADP and Pi.
-
Detachment: A new ATP molecule binds to the myosin head, causing it to detach from the actin filament.
-
ATP Hydrolysis and Cocking: The ATP bound to the myosin head is hydrolyzed to ADP and Pi. This hydrolysis provides the energy for the myosin head to return to its high-energy conformation, ready for another cycle. This repositioning of the myosin head is often referred to as "cocking."
Regulation Beyond Calcium: Other Influencing Factors
While calcium is the primary regulator, other factors influence the cross-bridge cycle and the binding of myosin to actin:
-
ATP availability: Without sufficient ATP, the myosin head cannot detach from actin, resulting in rigor mortis – the stiffening of muscles after death.
-
Phosphorylation: Phosphorylation of specific myosin light chains can modulate the myosin ATPase activity and cross-bridge kinetics.
-
Myosin isoforms: Different myosin isoforms, expressed in various muscle types, possess distinct kinetic properties, influencing the speed and force of contraction.
Implications of Disrupted Binding: Muscle Disorders
Disruptions in the binding of myosin to actin can lead to various muscle disorders. Mutations in genes encoding actin, myosin, tropomyosin, or troponin can compromise the cross-bridge cycle, resulting in:
-
Muscular dystrophies: A group of genetic disorders characterized by progressive muscle weakness and degeneration. Mutations affecting dystrophin, a protein connecting the actin cytoskeleton to the sarcolemma, can disrupt the structural integrity of muscle fibers, indirectly impacting the cross-bridge cycle.
-
Myopathies: A broad category of muscle diseases characterized by muscle weakness or wasting. Several myopathies arise from mutations affecting proteins involved in the cross-bridge cycle or muscle fiber organization.
Future Directions and Research
The field of muscle physiology continues to evolve, with ongoing research focused on:
-
Understanding the precise molecular mechanisms of cross-bridge interactions: High-resolution structural studies and advanced biophysical techniques are shedding light on the conformational changes during the cross-bridge cycle.
-
Developing novel therapeutic strategies for muscle disorders: Research is actively exploring potential therapies targeting the molecular defects underlying muscle diseases, aiming to restore normal muscle function.
-
Investigating the impact of aging and other factors on muscle function: Understanding how aging, exercise, and other factors affect the cross-bridge cycle is crucial for developing effective strategies to maintain muscle health throughout life.
Conclusion: A Symphony of Molecular Interactions
The binding of myosin cross-bridges to exposed sites on actin is a complex, highly regulated process central to muscle contraction. This interaction, orchestrated by calcium-dependent regulatory proteins, forms the basis for voluntary movement and countless other physiological processes. Disruptions in this tightly controlled binding can lead to a range of debilitating muscle disorders, highlighting the importance of continuing research into the intricacies of muscle physiology and its molecular mechanisms. Understanding the detailed interactions within this intricate dance remains a significant goal in scientific research, paving the way for future advancements in the treatment of muscle-related diseases and the enhancement of muscle function itself. Future research promises to unravel further mysteries, ultimately leading to a more comprehensive understanding of this fundamental biological process.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Lymphocyte
Apr 10, 2025
-
Correct Sequence Of Events In Phagocytosis
Apr 10, 2025
-
Ground State Electron Configuration For Aluminum
Apr 10, 2025
-
What Is The Difference Between Molar Mass And Molecular Mass
Apr 10, 2025
-
True Or False All Whole Numbers Are Integers
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about What Binds To The Exposed Cross Bridges On Actin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.