Using Osmotic Pressure To Find Molar Mass
News Leon
Apr 06, 2025 · 5 min read
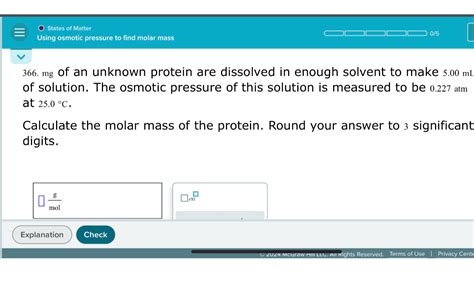
Table of Contents
Using Osmotic Pressure to Find Molar Mass: A Comprehensive Guide
Determining the molar mass of a solute is a fundamental task in chemistry, with applications ranging from pharmaceutical development to materials science. While several methods exist, osmotic pressure measurement offers a unique and powerful approach, particularly for large molecules like polymers or biological macromolecules. This detailed guide explores the principles behind this technique, its practical applications, and the calculations involved.
Understanding Osmotic Pressure
Osmotic pressure is a colligative property, meaning it depends on the concentration of solute particles but not their identity. It arises from the tendency of a solvent to move across a semipermeable membrane from a region of high solvent concentration (low solute concentration) to a region of low solvent concentration (high solute concentration). This movement continues until equilibrium is reached, characterized by a pressure difference across the membrane. This pressure difference is the osmotic pressure (π).
The Relationship Between Osmotic Pressure and Molar Mass
The van't Hoff equation elegantly connects osmotic pressure (π) to the molar concentration (c) of the solute, the ideal gas constant (R), and the absolute temperature (T):
π = cRT
However, this equation holds true only for ideal solutions, where solute-solute and solute-solvent interactions are negligible. For real solutions, particularly concentrated ones, deviations from ideality occur. The modified van't Hoff equation often incorporates an activity coefficient (γ) to account for these deviations:
π = γcRT
Since molar concentration (c) is related to molar mass (M) through the mass of the solute (m) and the volume of the solution (V): c = m/(MV), we can rewrite the equation to directly relate osmotic pressure to molar mass:
π = (m/MV)RT
Solving for molar mass (M):
M = mRT/(Vπ)
This equation provides a practical method for determining the molar mass of a solute by measuring its osmotic pressure.
Experimental Determination of Osmotic Pressure
Several experimental techniques can be employed to measure osmotic pressure. The most common methods include:
1. The Static Method
This classic method involves directly measuring the height of a solution column supported by the osmotic pressure. A semipermeable membrane separates the solution from the pure solvent. The height difference (h) between the two liquid levels is directly proportional to the osmotic pressure:
π = ρgh
where:
- ρ is the density of the solution
- g is the acceleration due to gravity
- h is the height difference
This method is simple in concept, but it can be time-consuming, and accurate measurements require careful control of temperature and pressure. It's also less suitable for solutions with low osmotic pressures, where the height difference is difficult to measure precisely.
2. The Dynamic Method
The dynamic method utilizes an apparatus that applies external pressure to counteract the osmotic pressure. The pressure required to stop the net flow of solvent across the membrane is directly equal to the osmotic pressure. This method offers greater accuracy and speed compared to the static method and is well-suited for measuring low osmotic pressures. Membrane osmometers are commonly used for this purpose.
3. Membrane Osmometers
Membrane osmometers are sophisticated instruments designed to accurately and efficiently measure osmotic pressure. They typically employ a dynamic method, applying precisely controlled pressure until the osmotic flow is stopped. These instruments are highly sensitive and capable of measuring osmotic pressures with high precision. They are frequently used in research and industrial applications.
Practical Considerations and Error Analysis
Several factors can influence the accuracy of molar mass determination using osmotic pressure:
-
Membrane Selection: The semipermeable membrane must be carefully chosen to be permeable to the solvent but impermeable to the solute. Membrane integrity and fouling can significantly affect the results.
-
Temperature Control: Temperature fluctuations can significantly impact osmotic pressure. Precise temperature control is crucial for accurate measurements.
-
Solution Preparation: Accurate preparation of the solution is paramount. The mass of the solute and the volume of the solution must be accurately measured. Solution homogeneity is also important to avoid concentration gradients.
-
Non-ideality: Deviations from ideal solution behavior can lead to errors in molar mass calculation. Using the modified van't Hoff equation with an activity coefficient can help mitigate this issue, although determining the activity coefficient itself can be challenging.
-
Calibration: Regular calibration of the osmometer is necessary to ensure accuracy.
Applications of Osmotic Pressure in Molar Mass Determination
The determination of molar mass using osmotic pressure finds widespread applications in various fields:
1. Polymer Chemistry
Determining the molar mass of polymers is crucial for understanding their properties and applications. Osmotic pressure measurement is particularly useful for polymers with high molar masses, where other techniques like vapor pressure osmometry may be less effective. The technique is employed in the characterization of synthetic polymers like polyethylene and natural polymers like proteins and carbohydrates.
2. Biochemistry and Biotechnology
Osmotic pressure is a valuable tool in biochemistry for characterizing biological macromolecules such as proteins, nucleic acids, and polysaccharides. It provides an effective way to determine the molar mass of these important molecules, assisting in protein purification and analysis.
3. Pharmaceutical Science
In pharmaceutical applications, osmotic pressure measurement helps in determining the molar mass of drugs and other pharmaceutical compounds. This is essential for understanding their properties, formulating appropriate drug delivery systems, and ensuring drug stability and efficacy.
4. Food Science
In the food industry, osmotic pressure is used to determine the molecular weight of food components, such as proteins and polysaccharides. This knowledge helps in optimizing food processing and ensuring the quality and stability of food products.
Conclusion
Osmotic pressure measurement offers a powerful and versatile method for determining the molar mass of various solutes, especially those with high molecular weights. While the experimental setup may require careful attention to detail, the resulting data provides valuable insights into the molecular characteristics of the sample. This understanding has implications across numerous scientific disciplines, from the development of new materials to the advancement of pharmaceutical therapies. The selection of the appropriate method (static or dynamic) and consideration of potential sources of error are crucial for achieving accurate and reliable results. With proper planning and execution, osmotic pressure measurements can provide significant insights into the molar mass of a solute, contributing to a deeper understanding of its properties and behavior.
Latest Posts
Latest Posts
-
What Is The Electron Configuration For Arsenic
Apr 08, 2025
-
The Elements Found In Chlorophyll Are
Apr 08, 2025
-
What Type Of Rock Makes Up The Continental Crust
Apr 08, 2025
-
What Is The Reciprocal Of 4 9
Apr 08, 2025
-
A Block Of Mass M Slides Between Two Rough Plates
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Using Osmotic Pressure To Find Molar Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.