The Types Of Bonds Found In Nucleic Acids Are
News Leon
Mar 20, 2025 · 6 min read
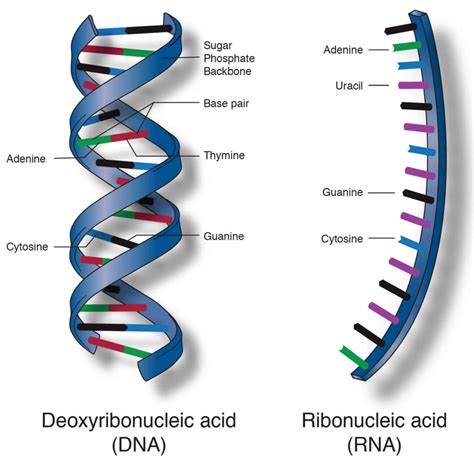
Table of Contents
The Types of Bonds Found in Nucleic Acids Are... A Deep Dive
Nucleic acids, the fundamental building blocks of life, are incredibly complex molecules responsible for storing and transmitting genetic information. Understanding the types of bonds that hold these molecules together is crucial to understanding their structure, function, and ultimately, the mechanisms of life itself. This article will delve into the various types of bonds found in nucleic acids, exploring their individual characteristics and the critical roles they play in the overall architecture and functionality of DNA and RNA.
The Backbone: Phosphodiester Bonds – Linking the Nucleotides
The backbone of both DNA and RNA is formed by a series of phosphodiester bonds. These bonds are covalent linkages that connect the 3' carbon atom of one sugar molecule (deoxyribose in DNA, ribose in RNA) to the 5' carbon atom of the next sugar molecule in the chain. This creates a sugar-phosphate backbone, with the nitrogenous bases projecting outwards.
Formation of Phosphodiester Bonds
The formation of a phosphodiester bond is a dehydration reaction. A hydroxyl group (-OH) from the 3' carbon of one nucleotide and a hydroxyl group from the phosphate group attached to the 5' carbon of another nucleotide react, releasing a water molecule (H₂O). This process leaves a phosphate group bridging the two sugars, forming the characteristic phosphodiester linkage.
Importance of Phosphodiester Bonds
The phosphodiester bonds are crucial because they:
- Create the structural framework: They provide the strength and stability necessary to maintain the long polynucleotide chains of DNA and RNA.
- Determine the directionality: The 5' to 3' directionality of nucleic acid strands is a direct consequence of the phosphodiester bond's formation. This directionality is essential for many biological processes, including DNA replication and transcription.
- Contribute to the overall charge: The phosphate groups carry a negative charge, giving the nucleic acid backbone an overall negative charge. This negative charge is important for interactions with proteins and other molecules.
The Base Pairing: Hydrogen Bonds – Holding the Double Helix Together
In DNA, the famous double helix structure is maintained by hydrogen bonds between complementary base pairs. These relatively weak bonds are essential for the stability of the DNA molecule, yet they are weak enough to allow for easy separation of the strands during processes like DNA replication and transcription.
Base Pairing Rules: Adenine with Thymine, Guanine with Cytosine
The hydrogen bonds in DNA follow specific base pairing rules:
- Adenine (A) forms two hydrogen bonds with Thymine (T).
- Guanine (G) forms three hydrogen bonds with Cytosine (C).
These specific pairings are due to the precise arrangement of hydrogen bond donor and acceptor atoms within the nitrogenous bases. The geometry of these bonds allows for a stable and relatively consistent distance between the two strands of the double helix.
Strength and Significance of Hydrogen Bonds in DNA
While individual hydrogen bonds are weak, the collective effect of numerous hydrogen bonds between base pairs along the DNA molecule contributes significantly to the overall stability of the double helix. The strength of the interaction is also influenced by the number of hydrogen bonds; G-C base pairs, with three hydrogen bonds, are generally stronger than A-T base pairs, with two hydrogen bonds. This difference in strength plays a role in determining the melting temperature (Tm) of DNA, the temperature at which the double helix denatures (separates into single strands).
In RNA, while hydrogen bonding is important for secondary structure formation (hairpins, loops, etc.), the pairing rules are slightly different. Adenine pairs with uracil (U) instead of thymine, while guanine still pairs with cytosine. RNA is typically single-stranded, although it can fold into complex three-dimensional structures through extensive intramolecular hydrogen bonding.
Beyond the Basics: Other Important Bonds in Nucleic Acid Structure and Function
While phosphodiester and hydrogen bonds are the most prominent types of bonds in nucleic acids, other interactions also contribute to their overall structure and function. These include:
1. Glycosidic Bonds: Linking Bases to Sugars
A glycosidic bond is a covalent bond that joins the nitrogenous base to the 1' carbon atom of the sugar (deoxyribose or ribose). This bond is crucial for connecting the bases to the sugar-phosphate backbone, completing the nucleotide unit. The specific type of glycosidic bond (α or β) influences the properties of the nucleotide and, consequently, the nucleic acid.
2. Hydrophobic Interactions: Stacking of Bases
The hydrophobic interactions between the stacked nitrogenous bases in the DNA double helix contribute significantly to the overall stability of the structure. The relatively nonpolar bases tend to cluster together in the center of the helix, minimizing their contact with water. This stacking effect is enhanced by van der Waals forces between the base pairs. The hydrophobic effect is also important in RNA folding, helping to determine the tertiary structure.
3. Ionic Interactions: Interactions with Metal Ions
Nucleic acids often interact with metal ions, such as magnesium (Mg²⁺), which can form ionic bonds with the negatively charged phosphate groups in the backbone. These interactions help to stabilize the nucleic acid structure and can influence its flexibility and conformation. Metal ions can also play a role in enzymatic activity involving nucleic acids.
4. Van der Waals Forces: Weak, but Cumulative Interactions
Van der Waals forces are weak, short-range attractive forces between molecules. While individually weak, the cumulative effect of numerous van der Waals interactions between base pairs, sugar molecules, and other components of the nucleic acid contributes to the overall stability of the molecule. These forces are especially significant in maintaining the close packing of bases in the DNA double helix and influencing the stability of RNA secondary structures.
5. Interactions with Proteins: Essential for Function
Nucleic acids rarely function in isolation. Their interactions with proteins are critical for numerous biological processes. These interactions can involve a variety of bonds, including hydrogen bonds, ionic interactions, and hydrophobic interactions. For example, DNA-binding proteins often recognize specific DNA sequences through hydrogen bonding and other interactions with the bases. These protein-nucleic acid interactions are essential for gene regulation, DNA replication, and repair.
The Importance of Understanding Nucleic Acid Bonding
Understanding the various types of bonds found in nucleic acids is fundamental to comprehending the intricacies of life. These bonds dictate the structure, stability, and function of DNA and RNA, ultimately shaping the processes of heredity, gene expression, and protein synthesis. The strength and specificity of these bonds allow for accurate replication, transcription, and translation of genetic information, ensuring the faithful transmission of hereditary traits across generations. Further research into the nuances of these bonds continues to reveal novel insights into biological mechanisms and potential therapeutic targets for various diseases. The sophisticated interplay of covalent and non-covalent bonds creates a dynamic yet robust system capable of storing and utilizing the genetic blueprint of all life. Appreciating the complexities of this molecular architecture underscores the elegance and precision of biological systems.
Latest Posts
Latest Posts
-
How Much Does A Drop Of Water Weigh
Mar 20, 2025
-
34 5 As A Mixed Number
Mar 20, 2025
-
The Size Of A Cell Is Limited By The
Mar 20, 2025
-
How Many Trips Around The Sun In A Year
Mar 20, 2025
-
What Is The Degree Of 9
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about The Types Of Bonds Found In Nucleic Acids Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
