The Specific Amino Acid Sequence Of A Protein Is Its
News Leon
Mar 24, 2025 · 7 min read
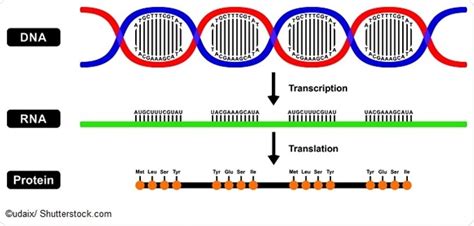
Table of Contents
The Specific Amino Acid Sequence of a Protein Is Its Primary Structure: A Deep Dive into Protein Structure and Function
The specific amino acid sequence of a protein is its primary structure. This seemingly simple statement underpins the entire complexity and functionality of proteins. Understanding primary structure, and its relationship to secondary, tertiary, and quaternary structures, is fundamental to grasping how proteins perform their myriad roles within living organisms. This article will delve deeply into the topic, exploring the implications of amino acid sequence on protein folding, function, and disease.
What is Primary Structure?
A protein's primary structure is the linear sequence of amino acids in a polypeptide chain. This sequence is dictated by the gene encoding the protein. Each amino acid is linked to the next by a peptide bond, a strong covalent bond formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of the next. This process, known as translation, occurs in ribosomes, utilizing the genetic information transcribed from DNA.
The order of amino acids is not random; it's precisely determined by the genetic code. A single change in this sequence – a substitution, deletion, or insertion of even one amino acid – can have significant consequences, altering the protein's shape, stability, and ultimately its function. This is crucial in understanding genetic diseases, many of which stem from mutations affecting the primary structure of proteins.
The Role of the Genetic Code
The genetic code, residing within DNA, is a blueprint for the construction of proteins. Each three-nucleotide sequence (codon) in mRNA specifies a particular amino acid. The sequence of codons in mRNA dictates the amino acid sequence during translation. This highly specific process ensures the accurate synthesis of proteins with precisely defined primary structures.
Importance of the N- and C-Terminus
The primary structure possesses directionality. One end of the polypeptide chain terminates with a free amino group (-NH2), designated as the N-terminus (amino-terminus), while the other end has a free carboxyl group (-COOH), known as the C-terminus (carboxyl-terminus). This directionality is critical for protein folding and function, influencing how the chain interacts with other molecules and itself.
From Primary to Higher-Order Structures
The primary structure isn't just a linear sequence; it dictates the higher-order structures that determine a protein's three-dimensional shape and function. The primary sequence contains inherent information that drives the folding process, leading to the formation of secondary, tertiary, and quaternary structures.
Secondary Structure: Local Folding Patterns
The primary structure folds locally into regular, repeating patterns, forming the secondary structure. These patterns are stabilized by hydrogen bonds between the amino and carboxyl groups of the polypeptide backbone. The most common secondary structures are:
-
α-helices: Right-handed coiled structures stabilized by hydrogen bonds between every fourth amino acid. The R-groups of amino acids project outwards from the helix. Certain amino acids are more prone to forming α-helices than others due to steric hindrance and charge interactions.
-
β-sheets: Extended polypeptide chains arranged side-by-side, forming a sheet-like structure. Hydrogen bonds stabilize the sheets, forming between adjacent chains. β-sheets can be parallel (chains run in the same direction) or anti-parallel (chains run in opposite directions). The R-groups of amino acids alternate above and below the plane of the sheet.
-
Turns and Loops: These are less ordered regions connecting α-helices and β-sheets. They are crucial for creating the overall three-dimensional shape of the protein and are often located on the protein's surface, allowing for interactions with other molecules.
Tertiary Structure: The 3D Arrangement
The overall three-dimensional arrangement of a polypeptide chain is its tertiary structure. This structure arises from interactions between amino acid side chains (R-groups) and the polypeptide backbone. These interactions include:
-
Hydrophobic interactions: Nonpolar amino acid side chains cluster together in the protein's interior, minimizing their contact with water.
-
Hydrogen bonds: Hydrogen bonds between polar side chains contribute to stability and shape.
-
Ionic bonds (salt bridges): Electrostatic attractions between oppositely charged side chains.
-
Disulfide bonds: Covalent bonds between cysteine residues, forming strong cross-links within the protein. These bonds are particularly important for stabilizing proteins that are secreted outside the cell.
The tertiary structure determines the protein's overall shape, creating specific pockets and surfaces that are crucial for its function. For example, enzymes often have active sites within their tertiary structure, precisely shaped to bind to substrates.
Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains, each with its own tertiary structure. The arrangement of these subunits is the quaternary structure. Interactions between subunits, similar to those in tertiary structure, stabilize the quaternary structure. Examples include hemoglobin, with its four subunits, and many other enzymes and structural proteins.
The Impact of Amino Acid Sequence on Protein Function
The primary sequence is not merely a list of amino acids; it is the foundation upon which all higher-order structures and functions are built. Slight changes in this sequence can have dramatic consequences:
-
Enzyme activity: The active site of an enzyme is precisely shaped by its tertiary structure, which is determined by its primary structure. Even a single amino acid substitution can disrupt the active site, reducing or eliminating enzymatic activity.
-
Protein-protein interactions: Proteins interact with each other to carry out many cellular processes. The surfaces of interacting proteins must complement each other in shape and charge. Alterations in primary structure can affect these interactions, disrupting signaling pathways or other vital processes.
-
Protein stability: The stability of a protein is crucial for its function. Certain amino acid sequences are more prone to forming stable structures than others. Mutations can destabilize proteins, making them susceptible to aggregation or degradation. This is a significant factor in many protein misfolding diseases.
-
Protein localization: The primary sequence often contains signals that direct the protein to its correct cellular location. These signals can be short amino acid sequences, and mutations in these regions can lead to mislocalization, disrupting protein function.
-
Protein-ligand interactions: Many proteins bind to specific molecules (ligands), such as hormones, neurotransmitters, or substrates. The primary sequence determines the three-dimensional structure that creates the binding site for these ligands. Mutations can disrupt binding, impairing cellular function.
Protein Misfolding and Disease
When proteins fail to fold correctly, they can lead to various diseases. The primary sequence is intrinsically linked to the correct folding pathway. Errors in this sequence can lead to misfolding, resulting in:
-
Loss of function: Misfolded proteins may lose their ability to perform their intended roles.
-
Aggregation: Misfolded proteins can aggregate, forming insoluble clumps that damage cells and tissues. This is a hallmark of neurodegenerative diseases like Alzheimer's and Parkinson's.
-
Dominant-negative effects: In some cases, a misfolded protein can interfere with the function of correctly folded proteins.
-
Gain of toxic function: Misfolded proteins can acquire new, harmful functions.
Many genetic diseases are linked to mutations that affect a protein's primary sequence, leading to misfolding and dysfunction. Understanding the relationship between primary structure and disease is crucial for developing effective therapies.
Conclusion: The Primary Structure as a Blueprint
The specific amino acid sequence of a protein is its primary structure—the fundamental blueprint for its existence. This sequence dictates every aspect of a protein's life, from its initial folding into complex three-dimensional shapes to its ultimate interaction with other molecules and its function within the cell. Even minor alterations in this sequence can have profound consequences, highlighting the exquisite precision of biological systems and the crucial role of primary structure in health and disease. Further research into the intricacies of protein folding and the impact of primary sequence alterations promises to lead to breakthroughs in various fields, from drug discovery to the treatment of genetic disorders. The more we understand this foundational principle, the better equipped we are to address a wide range of biological and medical challenges.
Latest Posts
Related Post
Thank you for visiting our website which covers about The Specific Amino Acid Sequence Of A Protein Is Its . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.