The Halogens Are The Most Reactive Among All The
News Leon
Mar 18, 2025 · 5 min read
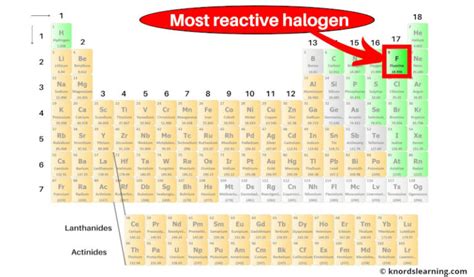
Table of Contents
The Halogens: The Most Reactive Nonmetals on the Periodic Table
The periodic table is a treasure trove of information, organizing elements based on their atomic structure and properties. Among these elements, the halogens stand out for their exceptional reactivity, making them fascinating and crucial elements in various fields, from everyday life to advanced technologies. This article delves deep into the world of halogens, exploring their unique properties, reactivity, reactions, and diverse applications.
Understanding the Halogen Family
The halogens, located in Group 17 (VIIA) of the periodic table, consist of five elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These elements share a common characteristic: they readily gain one electron to achieve a stable octet electron configuration, a feature that directly contributes to their high reactivity. This tendency to gain electrons makes them strong oxidizing agents.
Key Properties of Halogens
-
High Electronegativity: Halogens exhibit extremely high electronegativity, meaning they strongly attract electrons in a chemical bond. This property is a primary driver of their reactivity. Fluorine holds the distinction of being the most electronegative element.
-
Seven Valence Electrons: Each halogen atom has seven valence electrons, needing only one more to complete its outermost electron shell. This incomplete octet drives their eagerness to react with other elements.
-
Diatomic Molecules: In their elemental form, halogens exist as diatomic molecules (e.g., F₂, Cl₂, Br₂, I₂). This means two halogen atoms bond together to achieve stability.
-
Variable Oxidation States: Although their primary oxidation state is -1 (gaining one electron), halogens can exhibit various positive oxidation states in compounds, especially chlorine, bromine, and iodine. This versatility makes them involved in a wide array of chemical reactions.
-
Physical State Variation: Halogens display a fascinating range of physical states at room temperature: fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. This variation is due to the increasing strength of intermolecular forces with increasing atomic size.
The Reactivity of Halogens: A Deeper Dive
The extreme reactivity of halogens is a direct consequence of their high electronegativity and the need for one additional electron to complete their outermost electron shell. Their reactivity follows a trend: fluorine is the most reactive, followed by chlorine, bromine, iodine, and finally astatine.
Why is Fluorine the Most Reactive?
Several factors contribute to fluorine's exceptional reactivity:
-
Small Atomic Size: Fluorine's small atomic size results in a high electron density and strong attraction for electrons. The closer the incoming electron is to the nucleus, the stronger the attraction.
-
High Electronegativity: As mentioned earlier, fluorine has the highest electronegativity of all elements. This makes it exceptionally effective at attracting electrons from other atoms.
-
Weak F-F Bond: While the F-F bond is strong in the context of the other halogens, it's relatively weak compared to the bond strength predicted from atomic size and electronegativity. This weaker bond facilitates bond breaking and reactivity.
Reactivity Trends Down the Group
As you move down the halogen group, the reactivity decreases. This is attributed to several factors:
-
Increasing Atomic Size: Larger atoms have their outermost electrons further away from the nucleus, reducing the attraction between the nucleus and an incoming electron.
-
Decreasing Electronegativity: Electronegativity decreases down the group, diminishing the ability of the atom to attract electrons.
-
Increasing Electron Shielding: The increased number of inner electron shells in heavier halogens provides greater shielding of the outermost electrons from the nucleus, further weakening the attraction for incoming electrons.
Chemical Reactions of Halogens
Halogens readily participate in a wide array of chemical reactions, often exhibiting vigorous reactions with other elements and compounds.
Reactions with Metals
Halogens react vigorously with most metals, forming ionic compounds called halides. For example, sodium (Na) reacts violently with chlorine (Cl₂) to form sodium chloride (NaCl), common table salt:
2Na(s) + Cl₂(g) → 2NaCl(s)
The reaction is highly exothermic, releasing a significant amount of heat. Similar reactions occur with other metals and halogens.
Reactions with Nonmetals
Halogens also react with many nonmetals, forming covalent compounds. For example, chlorine reacts with hydrogen to form hydrogen chloride (HCl), a highly corrosive acid:
H₂(g) + Cl₂(g) → 2HCl(g)
The reactivity with nonmetals is generally less vigorous than with metals but still significant.
Displacement Reactions
Halogens can participate in displacement reactions, where a more reactive halogen displaces a less reactive one from its compound. For instance, chlorine can displace bromine from a bromide salt:
Cl₂(g) + 2KBr(aq) → 2KCl(aq) + Br₂(l)
This reaction illustrates the reactivity trend: chlorine, being more reactive than bromine, takes the place of bromine in the compound.
Applications of Halogens
Halogens and their compounds have numerous applications in various fields:
Industrial Applications
-
Chlorine: Used extensively in water treatment to disinfect and kill harmful microorganisms, in the production of plastics (PVC), and as a bleaching agent in paper and textile industries.
-
Fluorine: Crucial in the production of Teflon (polytetrafluoroethylene) and other fluoropolymers, which are known for their heat resistance and non-stick properties. It's also used in refrigerants and in toothpaste (fluoride).
Medical Applications
-
Iodine: Used as an antiseptic and disinfectant, often found in topical antiseptics and as a component of iodine-based contrast agents used in medical imaging.
-
Chlorine: Used in the production of various pharmaceuticals and disinfectants.
Other Applications
-
Bromine: Used as a flame retardant in plastics and textiles.
-
Astatine: Due to its radioactivity, astatine has limited applications, primarily in research related to nuclear medicine.
Environmental Concerns
While halogens have numerous benefits, their use also raises environmental concerns:
-
Ozone Depletion: Certain halogenated compounds, such as chlorofluorocarbons (CFCs), were found to deplete the ozone layer, leading to an international ban on their production and use.
-
Pollution: Improper handling and disposal of halogenated compounds can lead to soil and water contamination, posing risks to both human health and the environment.
Conclusion
The halogens represent a fascinating group of elements with unique and remarkable properties. Their high reactivity, stemming from their electronic structure and electronegativity, makes them essential components in numerous industrial, medical, and other applications. However, responsible use and disposal of halogens and their compounds are crucial to mitigate potential environmental risks and ensure their sustainable utilization. Further research and development in this field continue to uncover new applications and address the environmental challenges associated with their use, ensuring their valuable role in various aspects of our lives. Understanding their properties and reactions is crucial to both harnessing their benefits and mitigating their potential drawbacks. The continued study of halogens remains critical for scientific advancement and responsible environmental stewardship.
Latest Posts
Related Post
Thank you for visiting our website which covers about The Halogens Are The Most Reactive Among All The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.