The Central Part Of The Atom Is Called The
News Leon
Mar 20, 2025 · 6 min read
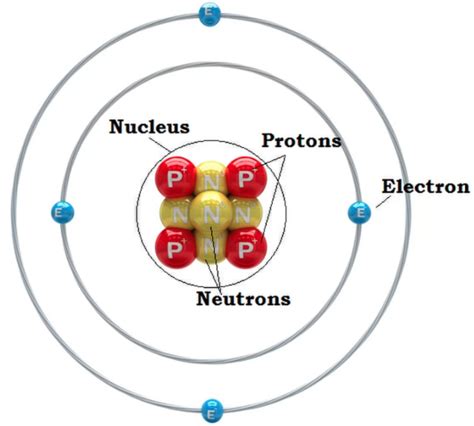
Table of Contents
The Central Part of the Atom is Called the Nucleus: A Deep Dive into Atomic Structure
The very heart of an atom, the entity that dictates its properties and behavior, is the nucleus. Understanding the nucleus is fundamental to grasping the intricacies of chemistry, physics, and even the workings of the universe itself. This article delves deep into the structure, composition, and significance of the atomic nucleus, exploring its crucial role in everything from the stability of matter to the power of nuclear energy.
Unveiling the Nucleus: A Subatomic World
The nucleus, residing at the atom's center, is incredibly small yet remarkably dense. It accounts for virtually all of the atom's mass, crammed into a space that is only a tiny fraction of the atom's overall volume. This concentration of mass is what gives the nucleus its immense significance.
Composition of the Nucleus: Protons and Neutrons
The nucleus is not a homogenous blob; instead, it is composed of two types of subatomic particles: protons and neutrons. These particles, collectively known as nucleons, are bound together by a powerful force – the strong nuclear force.
-
Protons: Positively charged particles carrying a single unit of positive charge. The number of protons in an atom's nucleus defines its atomic number and determines the element to which it belongs. For example, an atom with one proton is hydrogen, while an atom with six protons is carbon.
-
Neutrons: Electrically neutral particles, possessing no charge. They play a crucial role in stabilizing the nucleus. The number of neutrons in an atom's nucleus, along with the number of protons, determines the atom's mass number (total number of nucleons).
Isotopes: Variations in Neutron Number
Atoms of the same element can have different numbers of neutrons. These variations are known as isotopes. Isotopes of an element have the same number of protons (and thus the same atomic number) but different numbers of neutrons, resulting in different mass numbers. Some isotopes are stable, meaning their nuclei remain intact, while others are radioactive, undergoing spontaneous decay. This radioactive decay is often accompanied by the emission of particles and energy, a phenomenon exploited in various applications, including medical imaging and cancer treatment.
The Strong Nuclear Force: The Glue that Holds the Nucleus Together
The protons within the nucleus, all carrying positive charges, would naturally repel each other due to the electromagnetic force. However, the nucleus remains stable due to the strong nuclear force, a fundamental force far stronger than the electromagnetic force at short distances. This force overcomes the electrostatic repulsion between protons, holding the nucleons together tightly.
The strong nuclear force is a complex interaction mediated by particles called gluons. Its strength diminishes rapidly with distance, explaining why the strong force is only effective within the confines of the nucleus. Understanding the precise nature of the strong nuclear force is an ongoing area of research in particle physics.
Nuclear Stability and Radioactive Decay
Nuclear stability is a delicate balance between the strong nuclear force and the electromagnetic force. The ratio of neutrons to protons within the nucleus plays a critical role in determining an isotope's stability. For lighter elements, a roughly equal number of protons and neutrons often leads to stability. However, for heavier elements, a higher proportion of neutrons is needed to overcome the increased electrostatic repulsion between the greater number of protons.
When an isotope is unstable (radioactive), its nucleus undergoes spontaneous decay, transforming into a more stable configuration. This decay process can involve several different types of radiation:
- Alpha decay: Emission of an alpha particle (two protons and two neutrons).
- Beta decay: Emission of a beta particle (an electron or a positron).
- Gamma decay: Emission of a gamma ray (high-energy photon).
Radioactive decay is a random process, meaning it is impossible to predict precisely when a particular nucleus will decay. However, the rate of decay for a large number of nuclei follows a predictable pattern, characterized by a half-life. The half-life is the time it takes for half of the nuclei in a sample to decay. Half-lives can range from fractions of a second to billions of years.
Nuclear Fission and Fusion: Harnessing Nuclear Energy
The nucleus is not just a subject of scientific curiosity; it is also a source of immense energy. Two major processes exploit the energy stored within the nucleus:
-
Nuclear fission: The splitting of a heavy atomic nucleus into two lighter nuclei, releasing a large amount of energy. This process is utilized in nuclear power plants and nuclear weapons.
-
Nuclear fusion: The combining of two light atomic nuclei into a heavier nucleus, also releasing a significant amount of energy. This process powers the sun and other stars. Scientists are actively researching controlled fusion as a potential source of clean and virtually limitless energy.
Applications of Nuclear Science: From Medicine to Material Science
The study of the nucleus and its properties has led to numerous technological advancements across a variety of fields:
-
Medicine: Radioactive isotopes are used in medical imaging techniques such as PET (positron emission tomography) and SPECT (single-photon emission computed tomography) to diagnose diseases. Radiotherapy utilizes radioactive isotopes to target and destroy cancerous cells.
-
Material Science: Nuclear techniques are used to modify materials and improve their properties. For example, neutron irradiation can be used to enhance the strength and durability of certain materials.
-
Archaeology and Dating: Radioactive decay is used in radiocarbon dating to determine the age of ancient artifacts and organic materials.
-
Agriculture: Radioactive isotopes are used to study nutrient uptake in plants and improve crop yields.
Exploring the Frontier: Ongoing Research in Nuclear Physics
Despite decades of research, the nucleus continues to be a fascinating and challenging area of scientific inquiry. Scientists are continually refining our understanding of the strong nuclear force, exploring the behavior of exotic nuclei, and searching for new applications of nuclear technology. Research in nuclear physics pushes the boundaries of our knowledge, leading to breakthroughs in our understanding of the fundamental forces of nature and the universe's origins.
Further research focuses on:
-
Understanding the structure of exotic nuclei: Nuclei far from the line of stability, with unusual neutron-to-proton ratios, exhibit unique properties and offer valuable insights into nuclear forces.
-
Developing more efficient and sustainable nuclear energy sources: Research into controlled fusion remains a top priority, with the potential to revolutionize energy production.
-
Advancing nuclear medicine: Development of new radiotracers and radiotherapy techniques offers hope for improved cancer treatment and disease diagnosis.
-
Exploring applications of nuclear techniques in other scientific fields: Nuclear techniques are increasingly used in diverse fields such as environmental science, geology, and materials science.
Conclusion: The Nucleus – A Microcosm of the Universe
The nucleus, the central part of the atom, is a remarkable entity. Its composition, stability, and behavior dictate the properties of matter and the processes that shape the universe. From the stability of elements to the power of nuclear reactions, the nucleus plays a pivotal role in the physical world. Ongoing research continues to unveil the secrets of this tiny yet mighty component, promising further advancements in science and technology with far-reaching implications for our understanding of the cosmos and our place within it. The nucleus, therefore, remains a critical area of study, essential for advancing our knowledge across various scientific disciplines and technological applications. Its study will continue to uncover further wonders and possibilities in the years to come.
Latest Posts
Latest Posts
-
How Many Trips Around The Sun In A Year
Mar 20, 2025
-
What Is The Degree Of 9
Mar 20, 2025
-
Are Frogs Omnivores Herbivores Or Carnivores
Mar 20, 2025
-
How Many Years Is A Four Score
Mar 20, 2025
-
Length Of Perpendicular From A Point To A Line
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about The Central Part Of The Atom Is Called The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
