The Central Part Of An Atom Is Called The
News Leon
Mar 22, 2025 · 6 min read
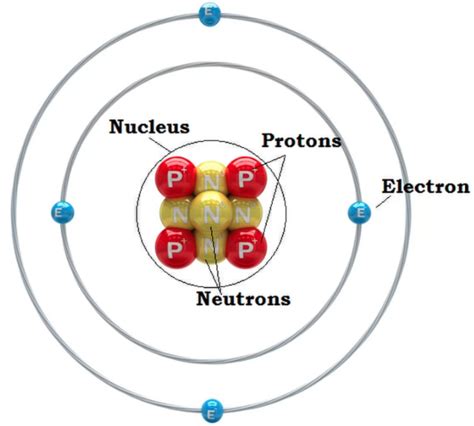
Table of Contents
The Central Part of an Atom is Called the Nucleus: A Deep Dive into Atomic Structure
The atom, the fundamental building block of matter, is a fascinating microcosm of physics. Understanding its structure is crucial to comprehending the properties of all substances, from the air we breathe to the stars in the sky. A central question in atomic studies is: What is the central part of an atom called? The answer, simply put, is the nucleus. But the story doesn't end there. The nucleus is a complex and crucial component, holding the key to an atom's identity and behavior. This article will delve deep into the structure and function of the atomic nucleus, exploring its composition, properties, and significance in various scientific fields.
Understanding the Atom's Architecture
Before diving into the specifics of the nucleus, let's briefly review the overall structure of an atom. An atom consists primarily of three subatomic particles:
- Protons: Positively charged particles found within the nucleus.
- Neutrons: Neutral (uncharged) particles also residing in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The vast majority of an atom's mass is concentrated in its nucleus, a tiny, dense region at the atom's center. The electrons, comparatively much lighter, occupy a significantly larger volume surrounding the nucleus. This arrangement, often depicted as a miniature solar system, gives the atom its characteristic size and properties. However, this is a simplified model; the actual behavior of electrons is far more complex, governed by the principles of quantum mechanics.
The Nucleus: A Closer Look
The atomic nucleus, that central core, is a powerhouse of positive charge and mass. Its composition and properties determine the atom's atomic number, mass number, and overall behavior. Let's explore its key aspects:
1. Composition: Protons and Neutrons
The nucleus is composed of protons and neutrons, collectively known as nucleons. These particles are held together by a powerful force called the strong nuclear force, which overcomes the electrostatic repulsion between the positively charged protons. Without this strong force, the nucleus would immediately fly apart.
-
Protons (p+): Each proton carries a single positive elementary charge, equal in magnitude but opposite in sign to the charge of an electron. The number of protons in an atom's nucleus defines its atomic number, which uniquely identifies the element. For example, hydrogen (H) has one proton (atomic number 1), helium (He) has two (atomic number 2), and so on.
-
Neutrons (n): Neutrons are electrically neutral, meaning they have no charge. Their presence in the nucleus contributes to the atom's mass number, which is the total number of protons and neutrons. The number of neutrons in an atom's nucleus can vary, even for the same element. These variations are known as isotopes. For example, carbon-12 has six protons and six neutrons, while carbon-14 has six protons and eight neutrons.
2. Nuclear Forces: What Holds It Together?
The strong nuclear force is one of nature's four fundamental forces. It's incredibly powerful, acting over a very short range within the nucleus. This force is responsible for binding protons and neutrons together, despite the electrostatic repulsion between protons. The strength of this force is essential for the stability of the nucleus.
Another important force at play is the weak nuclear force, which is responsible for radioactive decay. This force mediates certain types of nuclear transformations, resulting in the emission of particles like beta particles and neutrinos.
3. Nuclear Size and Density: Tiny but Mighty
The nucleus is exceptionally small, occupying only a tiny fraction of the atom's overall volume. Despite its diminutive size, it possesses an incredibly high density, packing a substantial amount of mass into an extremely small space. This density is far greater than anything found in ordinary matter.
4. Nuclear Stability and Isotopes
The stability of an atomic nucleus depends on the balance between the strong nuclear force and the electrostatic repulsion between protons. Nuclei with a favorable ratio of protons to neutrons are generally stable. However, some nuclei are unstable and undergo radioactive decay to reach a more stable configuration.
Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. Some isotopes are stable, while others are radioactive, undergoing decay processes that emit particles and energy. Radioactive isotopes have various applications in medicine, archaeology, and industry.
Significance of the Nucleus in Science and Technology
The nucleus, the central part of an atom, plays a crucial role in various scientific fields and technological applications:
1. Nuclear Physics and Energy: Harnessing Nuclear Power
The study of the nucleus and its properties forms the basis of nuclear physics. Our understanding of nuclear forces and radioactive decay has led to the development of nuclear power, providing a significant source of energy. However, it's crucial to acknowledge the challenges and risks associated with nuclear technology, including the management of nuclear waste and the potential for accidents.
2. Nuclear Medicine: Fighting Disease with Isotopes
Radioactive isotopes have revolutionized medical diagnosis and treatment. Techniques like PET (Positron Emission Tomography) scans use radioactive tracers to visualize internal organs and detect diseases like cancer. Radiotherapy utilizes radiation emitted by radioactive isotopes to target and destroy cancerous cells.
3. Nuclear Dating: Unraveling the Past
Radioactive decay provides a powerful tool for dating ancient artifacts and geological formations. By measuring the ratio of isotopes in a sample, scientists can determine its age, offering insights into the Earth's history and the development of human civilization.
4. Nuclear Chemistry: Transforming Matter
Nuclear reactions can transform one element into another, a process known as transmutation. This capability has applications in producing new elements, synthesizing isotopes for research, and creating radioactive tracers for various purposes.
5. Material Science: Enhancing Properties
The properties of materials are intimately related to the structure of their constituent atoms and their nuclei. Manipulating the nuclear properties of materials can lead to the development of novel materials with improved strength, durability, and other desirable characteristics.
Conclusion: The Nucleus – A Tiny Powerhouse
In conclusion, the central part of an atom is called the nucleus. This tiny, dense region is far more than just a central point; it's the heart of the atom, dictating its identity, mass, and behavior. Composed of protons and neutrons held together by the strong nuclear force, the nucleus governs atomic number, mass number, and isotopic variations. Our understanding of the nucleus has revolutionized numerous fields, from energy production and medicine to archaeology and material science. While its scale is minuscule, its impact on our understanding of the universe and our technology is immense. Continued research into nuclear physics promises further advancements and applications in the years to come. The exploration of the nucleus remains a vital area of scientific inquiry, continually revealing new insights into the fundamental building blocks of our universe.
Latest Posts
Latest Posts
-
How Many Significant Digits Are In 100
Mar 23, 2025
-
What Is The Unit Of Moment Of Inertia
Mar 23, 2025
-
What Is The Complementary Strand Of Dna
Mar 23, 2025
-
Is Copper Hydroxide Soluble In Water
Mar 23, 2025
-
Relationship In Which Both Organisms Benefit
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about The Central Part Of An Atom Is Called The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
