The Backbone Of A Nucleic Acid Strand Is Composed Of
News Leon
Mar 20, 2025 · 6 min read
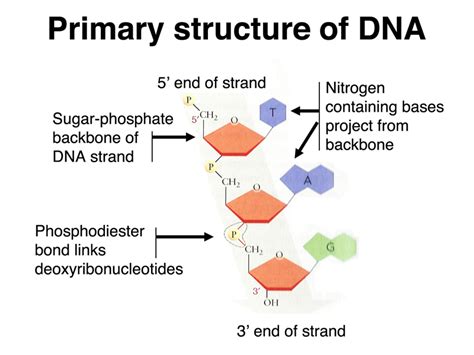
Table of Contents
The Backbone of a Nucleic Acid Strand: Deoxyribose and Ribose Sugars
The backbone of a nucleic acid strand—the structural framework that supports the genetic information—is a crucial component of DNA and RNA. Understanding its composition is fundamental to grasping the intricate mechanisms of heredity, gene expression, and countless biological processes. This backbone isn't just a passive scaffold; its chemical properties influence the stability, flexibility, and overall function of nucleic acids. This in-depth exploration will dissect the backbone's composition, highlighting the differences between DNA and RNA, and examining its significance in molecular biology.
The Sugar-Phosphate Backbone: A Detailed Look
Both DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) share a fundamental structural similarity: a sugar-phosphate backbone. This backbone is formed by the alternating sequence of sugar molecules and phosphate groups. However, the specific type of sugar differs between DNA and RNA, leading to significant functional variations.
The Role of Deoxyribose in DNA
In DNA, the sugar component is deoxyribose. This pentose sugar (a five-carbon sugar) lacks a hydroxyl (-OH) group at the 2' carbon position, hence the "deoxy" prefix. This seemingly minor difference has profound implications for DNA's stability and function. The absence of the 2'-OH group makes the DNA backbone less susceptible to hydrolysis (breakdown by water), contributing to its remarkable stability, crucial for the long-term storage of genetic information.
-
Chemical Structure: Deoxyribose has a ring structure composed of four carbons and one oxygen atom. The carbon atoms are numbered 1' to 5', with the 5' carbon bonded to a phosphate group and the 1' carbon attached to a nitrogenous base.
-
Stability: The lack of the 2'-OH group in deoxyribose reduces the reactivity of the DNA backbone, making it less prone to degradation compared to RNA. This increased stability is essential for maintaining the integrity of the genome across generations.
-
Conformation: The deoxyribose sugar's specific conformation contributes to the double-helical structure of DNA. The orientation of its atoms influences the way the bases interact, forming the characteristic base pairing crucial for DNA replication and transcription.
The Role of Ribose in RNA
In RNA, the sugar component is ribose, a pentose sugar identical to deoxyribose except for the presence of a hydroxyl (-OH) group at the 2' carbon position. This seemingly small difference has significant consequences for RNA's properties and functions.
-
Chemical Structure: Ribose, like deoxyribose, is a pentose sugar with a ring structure. However, it possesses a hydroxyl group at the 2' carbon, which is absent in deoxyribose.
-
Reactivity: The presence of the 2'-OH group in ribose increases RNA's reactivity compared to DNA. This hydroxyl group can participate in chemical reactions, such as hydrolysis, making RNA less stable than DNA. This instability is, paradoxically, a key feature for its function as a transient information carrier. RNA's shorter lifespan allows for more dynamic regulatory roles.
-
Structure and Function: The presence of the 2'-OH group influences RNA's secondary structure. It can participate in hydrogen bonding, contributing to the formation of complex three-dimensional structures essential for RNA's diverse catalytic and regulatory roles. The flexibility conferred by the 2'-OH group also contributes to the ability of certain RNA molecules to fold into highly specific shapes for their functions.
The Phosphate Group: Linking the Sugars
The phosphate group is the other crucial component of the nucleic acid backbone. It acts as a bridge, linking the 3' carbon of one sugar molecule to the 5' carbon of the next sugar molecule in the chain. This creates a repeating sugar-phosphate-sugar-phosphate pattern, forming the backbone's characteristic structure.
-
Phosphodiester Bond: The linkage between the phosphate group and the sugars is called a phosphodiester bond. This bond is a strong covalent bond, providing structural integrity to the backbone. The formation of this bond involves the removal of a water molecule, a dehydration reaction.
-
Negative Charge: The phosphate group carries a negative charge at physiological pH. This negative charge contributes to the hydrophilic nature of the nucleic acid backbone, making it soluble in water and interacting with positively charged ions and proteins. The negative charge also contributes to the overall stability of the double helix in DNA. Repulsion between the negatively charged phosphate groups contributes to the helical twist and prevents the collapse of the double helix.
-
Directionality: The phosphodiester bond establishes a directionality in the nucleic acid chain. The chain has a 5' end (the end with the free 5' phosphate group) and a 3' end (the end with the free 3' hydroxyl group). This directionality is crucial for DNA replication, transcription, and translation. Enzymes involved in these processes recognize and act upon the specific ends of the nucleic acid strand.
Differences Between DNA and RNA Backbones: Implications for Function
The differences between the deoxyribose and ribose sugars have significant consequences for the properties and functions of DNA and RNA.
| Feature | DNA (Deoxyribose) | RNA (Ribose) |
|---|---|---|
| Sugar | Deoxyribose (lacks 2'-OH group) | Ribose (has 2'-OH group) |
| Stability | More stable (resistant to hydrolysis) | Less stable (prone to hydrolysis) |
| Reactivity | Less reactive | More reactive |
| Structure | Typically double-stranded helix | Typically single-stranded, can form complex secondary structures |
| Function | Long-term storage of genetic information | Transient information carrier, catalytic and regulatory roles |
The greater stability of DNA is crucial for its role as the long-term repository of genetic information. The double helix structure protects the bases from chemical modification and degradation. In contrast, the increased reactivity and flexibility of RNA allows it to perform a wide variety of dynamic functions, including catalysis (ribozymes), gene regulation (microRNAs, siRNAs), and protein synthesis (mRNA, tRNA, rRNA).
The Backbone's Influence on Nucleic Acid Interactions
The sugar-phosphate backbone isn't just a structural scaffold; it actively participates in interactions that are essential for nucleic acid function.
-
Protein Binding: The negatively charged phosphate groups interact with positively charged amino acid residues in proteins. This electrostatic interaction is crucial for the binding of various proteins to DNA and RNA, including enzymes involved in replication, transcription, and repair. Specific patterns of phosphate interactions can determine the selectivity of protein binding.
-
DNA Bending and Supercoiling: The flexibility of the backbone allows DNA to bend and supercoil. This is important for packaging DNA into chromosomes, as well as regulating gene expression through interactions with regulatory proteins.
-
RNA Folding: The flexibility of the RNA backbone allows it to fold into complex three-dimensional structures. These structures are crucial for the catalytic and regulatory functions of many RNA molecules.
Conclusion: The Backbone's Central Role
The sugar-phosphate backbone is not a mere supporting structure but a dynamic component that profoundly influences the properties and functions of nucleic acids. The differences between DNA's deoxyribose backbone and RNA's ribose backbone highlight the exquisite adaptation of these molecules to their respective roles in the cell. Understanding the backbone's chemical composition and its influence on nucleic acid interactions is fundamental to comprehending the intricate machinery of life. Future research into the intricacies of the nucleic acid backbone promises to unveil further insights into the complexities of genetics, molecular biology, and the evolution of life itself. From the stability of the genetic code to the dynamic versatility of RNA, the backbone lays the foundation for the remarkable functions of these essential biomolecules.
Latest Posts
Latest Posts
-
Which Of The Following Are Powerful Vasoconstrictors
Mar 21, 2025
-
What Is The Gram Formula Mass Of Ca Oh 2
Mar 21, 2025
-
2 X 1 X 2 Integral
Mar 21, 2025
-
What Qualities Make A Good Leader Essay
Mar 21, 2025
-
Character Sketch Of Helen Keller For 10 Marks
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about The Backbone Of A Nucleic Acid Strand Is Composed Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
