Select The Correct Iupac Name For The Compound Shown
News Leon
Mar 29, 2025 · 6 min read
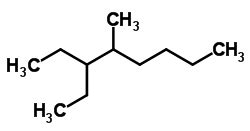
Table of Contents
Selecting the Correct IUPAC Name for Organic Compounds: A Comprehensive Guide
Naming organic compounds correctly is a cornerstone of organic chemistry. The International Union of Pure and Applied Chemistry (IUPAC) provides a systematic nomenclature that allows chemists worldwide to unambiguously identify and communicate about millions of different molecules. This article will delve into the process of selecting the correct IUPAC name, exploring the fundamental rules and offering practical examples to solidify understanding. Mastering this skill is crucial for anyone studying or working in the field of chemistry.
Understanding the IUPAC System: A Foundation for Nomenclature
The IUPAC system is based on a set of logical rules that prioritize clarity and consistency. It eliminates ambiguity by assigning a unique name to each organic compound based on its structure. The core principles revolve around identifying the parent chain, functional groups, substituents, and their positions within the molecule. Let's explore these key components:
1. Identifying the Parent Chain: The Backbone of the Molecule
The parent chain is the longest continuous chain of carbon atoms in the molecule. This forms the foundation upon which the entire name is built. When multiple chains of equal length exist, the one with the most substituents is selected as the parent. Consider a molecule like 2-methylpentane. 'Pentane' indicates the five-carbon parent chain.
2. Recognizing Functional Groups: Defining the Compound's Nature
Functional groups are specific groups of atoms within a molecule that are responsible for its characteristic chemical reactions and properties. These groups are given priority in the naming process. Examples include alcohols (-OH), ketones (C=O), aldehydes (-CHO), carboxylic acids (-COOH), and amines (-NH2). The presence of a functional group significantly influences the name. For instance, the presence of an -OH group designates the compound as an alcohol.
3. Locating and Naming Substituents: Branching Out from the Parent Chain
Substituents are atoms or groups of atoms attached to the parent chain. They are named according to their structure and position on the chain. The position is indicated by a number, with numbering starting from the end of the parent chain that gives the substituents the lowest possible numbers. Consider 3-methylhexane; the methyl group (-CH3) is attached to the third carbon atom of the hexane chain.
4. Applying IUPAC Rules: A Step-by-Step Approach
To select the correct IUPAC name, follow these steps methodically:
- Identify the Parent Chain: Determine the longest continuous carbon chain.
- Identify the Functional Group: Determine the highest priority functional group present. This often dictates the suffix of the name (e.g., -ol for alcohols, -one for ketones).
- Identify and Number Substituents: Number the carbon atoms of the parent chain, assigning the lowest possible numbers to the substituents.
- Name Substituents: Name each substituent alphabetically, ignoring prefixes like di, tri, etc., when alphabetizing.
- Assemble the Name: Combine the substituent names (with their positions), the parent chain name, and the functional group suffix to create the complete IUPAC name.
Practical Examples: Applying the Rules to Different Compounds
Let's analyze several examples to solidify our understanding:
Example 1: A Simple Alkane
Consider the molecule with the structure: CH3-CH2-CH(CH3)-CH2-CH3.
- Parent Chain: The longest continuous chain contains five carbons, making it pentane.
- Functional Group: There is no functional group other than the single bonds.
- Substituents: A methyl group (-CH3) is attached to the third carbon.
- Name: 3-Methylpentane
Example 2: An Alcohol
Consider the molecule with the structure: CH3-CH(OH)-CH2-CH3.
- Parent Chain: The longest continuous chain is four carbons (butane).
- Functional Group: A hydroxyl group (-OH) is present, indicating an alcohol.
- Substituents: The hydroxyl group is on the second carbon.
- Name: 2-Butanol
Example 3: A Ketone
Consider the molecule with the structure: CH3-CO-CH2-CH3.
- Parent Chain: Four carbons, butane.
- Functional Group: A ketone group (C=O). The carbonyl group is on the second carbon.
- Substituents: None.
- Name: Butan-2-one (or 2-Butanone, both acceptable).
Example 4: A More Complex Molecule
Let's consider a more complex example: CH3-CH(CH3)-CH(Cl)-CH2-CH3.
- Parent Chain: Five carbons, pentane.
- Functional Group: None (alkane).
- Substituents: A methyl group on carbon 2 and a chloro group (-Cl) on carbon 3. We number to give the lowest possible sum of numbers for the substituents (2+3=5 is preferable to 2+4=6)
- Name: 2-Methyl-3-chloropentane (Methyl comes before chloro alphabetically).
Example 5: Dealing with Multiple Substituents
Let's analyze CH3-CH(CH3)-CH(C2H5)-CH2-CH3.
- Parent Chain: Five carbons, pentane.
- Functional Group: None (alkane).
- Substituents: An ethyl group (-CH2CH3) on carbon 3 and a methyl group on carbon 2.
- Name: 2-Methyl-3-ethylpentane (Ethyl comes before methyl alphabetically)
Example 6: Cyclic Compounds
The naming of cyclic compounds involves similar principles but with a few additional considerations:
Consider a cyclohexane ring with a methyl group and a chlorine atom:
- Parent Chain: Cyclohexane.
- Functional Group: None (alkane).
- Substituents: Methyl and chloro group. Numbering is chosen to give the lowest possible locant numbers, considering both substituents
- Name: 1-Chloro-3-methylcyclohexane (Or 3-Chloro-1-methylcyclohexane which is considered equivalent)
Advanced Concepts in IUPAC Nomenclature
While the above examples cover fundamental concepts, more complex scenarios exist. These include:
- Multiple identical substituents: Prefixes like di, tri, tetra etc. are used. These are also used to number the position of multiple identical substituents (e.g. 2,3-dimethylbutane).
- Complex substituents: Substituents themselves can have branches and need to be named recursively following the same rules. These are often named as alkyl groups.
- Unsaturated compounds: Alkenes (C=C) and alkynes (C≡C) require specifying the position of the multiple bonds in the name.
- Stereoisomers: IUPAC names also specify the stereochemistry (cis/trans, E/Z) when relevant.
Conclusion: Mastering IUPAC Nomenclature for Success in Chemistry
The ability to correctly name organic compounds according to IUPAC rules is vital for accurate communication and comprehension in chemistry. This article provided a foundation in the naming process, outlining the steps involved in identifying the parent chain, functional groups, and substituents, and assembling the complete name. By diligently applying these rules and practicing with diverse examples, students and professionals can enhance their understanding of organic chemistry and improve their ability to navigate the complexities of organic nomenclature. The more you practice, the more intuitive this system will become, and the more confident you'll be in selecting the correct IUPAC name for any organic compound you encounter. Remember to consult detailed IUPAC guidelines for nuanced cases and to ensure complete accuracy.
Latest Posts
Latest Posts
-
How Do The Daughter Cells Compare To The Parent Cell
Apr 01, 2025
-
A Group Of Closely Related Species Is A
Apr 01, 2025
-
Which Of The Is Not A Greenhouse Gas
Apr 01, 2025
-
How Many Moles In One Liter Of Water
Apr 01, 2025
-
Why Are Human Sex Hormones Considered Lipids
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Select The Correct Iupac Name For The Compound Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
