Reaction Of Salicylic Acid With Methanol
News Leon
Apr 01, 2025 · 5 min read
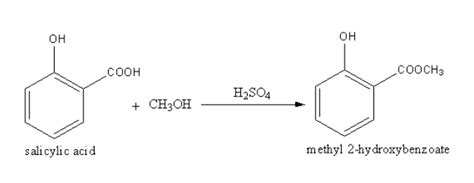
Table of Contents
Reaction of Salicylic Acid with Methanol: A Comprehensive Guide
The reaction of salicylic acid with methanol is a classic example of an esterification reaction, specifically the synthesis of methyl salicylate. This reaction, catalyzed by an acid, transforms the carboxylic acid group of salicylic acid into a methyl ester, resulting in a fragrant compound commonly found in wintergreen oil. This article delves into the intricacies of this reaction, covering its mechanism, reaction conditions, applications, and safety precautions.
Understanding the Reaction: Esterification
Esterification is a reversible reaction where a carboxylic acid reacts with an alcohol to form an ester and water. In the case of salicylic acid and methanol, the reaction can be represented as follows:
Salicylic Acid + Methanol ⇌ Methyl Salicylate + Water
The equilibrium of this reaction lies towards the reactants. To drive the reaction towards the product formation, we employ Le Chatelier's principle. This involves several strategies, primarily focusing on removing the water produced or using an excess of one of the reactants.
The Mechanism of Esterification: A Step-by-Step Breakdown
The acid-catalyzed esterification of salicylic acid with methanol proceeds via a nucleophilic acyl substitution mechanism. The steps are as follows:
-
Protonation of the Carboxylic Acid: The acid catalyst (typically concentrated sulfuric acid or hydrochloric acid) protonates the carbonyl oxygen of salicylic acid. This increases the electrophilicity of the carbonyl carbon, making it more susceptible to nucleophilic attack.
-
Nucleophilic Attack by Methanol: The oxygen atom of methanol, acting as a nucleophile, attacks the electrophilic carbonyl carbon. This forms a tetrahedral intermediate.
-
Proton Transfer: A proton transfer occurs within the tetrahedral intermediate, leading to the formation of a new hydroxyl group and a protonated methoxy group.
-
Elimination of Water: A molecule of water is eliminated from the intermediate. This step is facilitated by the acid catalyst, which removes a proton from the hydroxyl group, making it a better leaving group.
-
Deprotonation: The final step involves the deprotonation of the protonated ester by a base (often the conjugate base of the acid catalyst). This yields the methyl salicylate ester and regenerates the acid catalyst.
This mechanism highlights the crucial role of the acid catalyst in facilitating the reaction by activating the carbonyl group and assisting in the elimination of water.
Reaction Conditions: Optimizing the Synthesis
Several factors influence the yield and efficiency of methyl salicylate synthesis. Optimizing these conditions is vital for achieving a successful reaction.
Choice of Catalyst: The Importance of Acidic Conditions
The choice of acid catalyst significantly impacts the reaction rate and yield. Concentrated sulfuric acid is a common choice due to its high acidity and effectiveness in protonating the carboxylic acid. However, its corrosive nature necessitates careful handling. Hydrochloric acid can be a safer alternative, although it might require longer reaction times.
Temperature Control: Balancing Reaction Rate and Product Degradation
Temperature plays a crucial role in the reaction rate. Higher temperatures generally accelerate the reaction, but excessive heat can lead to side reactions or product degradation. A temperature range of 60-80°C is generally considered optimal for this reaction. Careful temperature monitoring is crucial to prevent unwanted side products.
Reaction Time: Reaching Equilibrium
The reaction time depends on several factors, including the catalyst used, the temperature, and the concentration of reactants. Sufficient reaction time is necessary to allow the reaction to reach equilibrium. Monitoring the reaction progress, for instance, using thin-layer chromatography (TLC), can help determine the optimal reaction time.
Reactant Ratios: Driving the Equilibrium
According to Le Chatelier's principle, using an excess of one reactant can shift the equilibrium toward the products. Typically, an excess of methanol is used to enhance the yield of methyl salicylate. This helps to drive the equilibrium to the right, favoring product formation.
Purification and Characterization of Methyl Salicylate
After the reaction is complete, the crude product needs to be purified to remove unreacted starting materials, catalyst, and any side products. Common purification techniques include:
-
Extraction: This involves separating the organic layer containing methyl salicylate from the aqueous layer containing water and the acid catalyst. A separatory funnel is typically used.
-
Washing: The organic layer is washed with water or a dilute base to remove any residual acid catalyst.
-
Drying: The organic layer is dried using an anhydrous drying agent like magnesium sulfate to remove any remaining water.
-
Distillation: Distillation is used to purify the methyl salicylate by separating it based on its boiling point.
Characterizing the purified methyl salicylate involves several techniques:
-
Gas Chromatography (GC): This is used to determine the purity of the obtained product.
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy confirms the structure of the synthesized compound.
-
Infrared (IR) Spectroscopy: IR spectroscopy provides information about the functional groups present in the molecule.
Applications of Methyl Salicylate: A Versatile Compound
Methyl salicylate boasts a wide array of applications, ranging from pharmaceuticals to cosmetics. Its distinctive odor and properties make it a valuable compound in several industries:
-
Flavoring Agent: It's extensively used as a flavoring agent in foods, beverages, and candies, particularly for its characteristic wintergreen flavor.
-
Fragrance Ingredient: In the cosmetic and fragrance industry, it serves as a key ingredient in perfumes, lotions, and other personal care products.
-
Pharmaceutical Applications: Methyl salicylate possesses analgesic and anti-inflammatory properties. It is often included in topical pain relief ointments and balms.
-
Insect Repellent: Some studies have shown that methyl salicylate displays repellent properties against certain insects.
-
Industrial Applications: It finds use as a solvent in various industrial applications and as an intermediate in the synthesis of other chemicals.
Safety Precautions: Handling Chemicals Safely
Handling chemicals, especially concentrated acids, requires careful attention to safety procedures:
-
Protective Equipment: Wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat.
-
Ventilation: Work in a well-ventilated area or under a fume hood to prevent inhalation of harmful vapors.
-
Acid Handling: Handle concentrated acids with extreme caution. Always add acid to water slowly and carefully, never the other way around, to prevent splashing and potential burns.
-
Waste Disposal: Dispose of chemical waste according to the appropriate safety guidelines and regulations.
Conclusion: A Valuable Synthetic Route
The synthesis of methyl salicylate from salicylic acid and methanol is a simple yet instructive experiment that illustrates the fundamental principles of esterification. Understanding the reaction mechanism, optimizing the reaction conditions, and employing appropriate purification and characterization techniques are crucial for a successful synthesis. The wide range of applications of methyl salicylate further emphasizes the importance of this reaction in various industries. Always remember to prioritize safety when working with chemicals.
Latest Posts
Latest Posts
-
Complete The Complementary Strand Of Dna
Apr 02, 2025
-
Is Carbon Dioxide A Pure Substance Or Mixture
Apr 02, 2025
-
The Given Reaction Proceeds In Two Parts
Apr 02, 2025
-
Which Of The Following Combinations Is Correct
Apr 02, 2025
-
How Do You Find The Boiling Point Of A Solution
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Reaction Of Salicylic Acid With Methanol . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
