Oxidation Number Of C In C2o42-
News Leon
Apr 12, 2025 · 6 min read
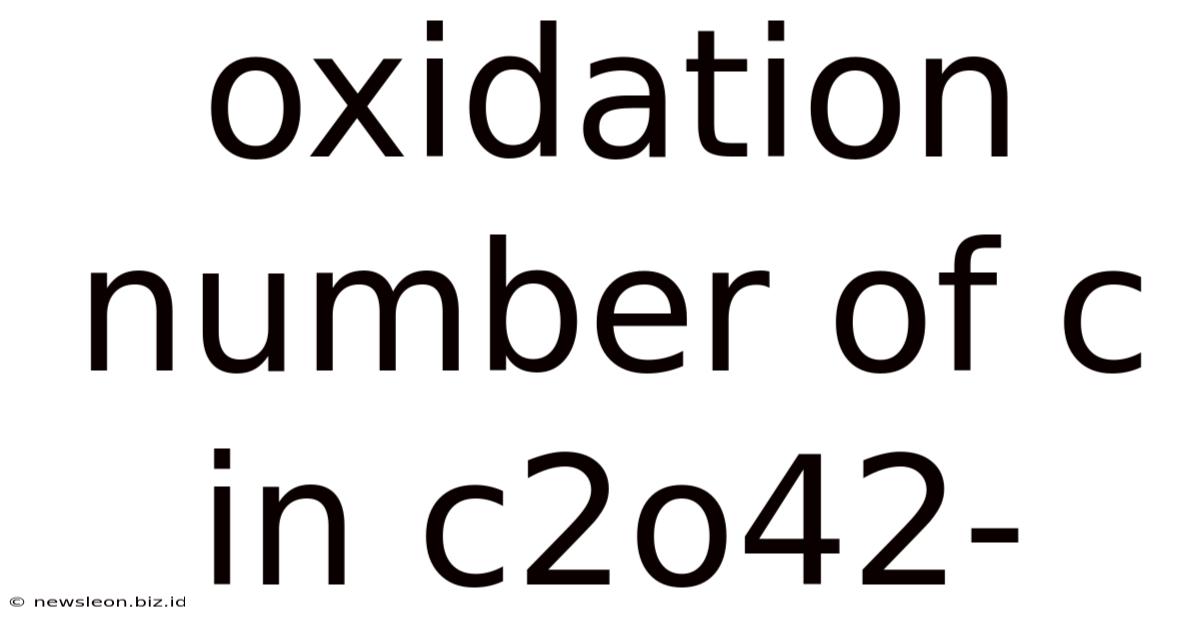
Table of Contents
Determining the Oxidation Number of Carbon in C₂O₄²⁻
The oxalate ion, C₂O₄²⁻, is a common ligand in coordination chemistry and an important intermediate in various metabolic processes. Understanding its structure and, specifically, the oxidation number of carbon within it, is crucial for comprehending its reactivity and role in chemical reactions. This article delves deep into the determination of the oxidation number of carbon in C₂O₄²⁻, exploring the methods involved and addressing common misconceptions.
Understanding Oxidation Numbers
Before tackling the specific case of C₂O₄²⁻, let's refresh the concept of oxidation numbers. An oxidation number, also known as an oxidation state, is a hypothetical charge assigned to an atom in a molecule or ion, assuming that all bonds are completely ionic. This is a useful tool for bookkeeping electrons and predicting the behavior of elements in chemical reactions. It's important to remember that oxidation numbers are not necessarily the actual charges on atoms, especially in covalent compounds where electron sharing is significant.
The rules for assigning oxidation numbers are as follows:
- Rule 1: The oxidation number of an element in its free (uncombined) state is zero. For example, the oxidation number of O₂ is 0, and the oxidation number of Fe(s) is 0.
- Rule 2: The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
- Rule 3: The oxidation number of hydrogen is +1, except in metal hydrides where it is -1.
- Rule 4: The oxidation number of oxygen is -2, except in peroxides (like H₂O₂) where it is -1 and in superoxides where it is -1/2.
- Rule 5: The oxidation number of a neutral molecule is always zero. The sum of the oxidation numbers of all atoms in a neutral molecule equals zero.
- Rule 6: The oxidation number of a polyatomic ion is equal to its overall charge. The sum of oxidation numbers of all atoms in a polyatomic ion equals the charge on the ion.
These rules provide a systematic approach to determining oxidation numbers, but some exceptions might arise in complex molecules.
Calculating the Oxidation Number of Carbon in C₂O₄²⁻
Now, let's apply these rules to determine the oxidation number of carbon in the oxalate ion, C₂O₄²⁻.
-
Identify the known oxidation numbers: We know that the oxidation number of oxygen is typically -2 (Rule 4).
-
Set up an equation: Let 'x' represent the oxidation number of carbon. Since there are two carbon atoms and four oxygen atoms in C₂O₄²⁻, we can write the equation:
2x + 4(-2) = -2
-
Solve for x:
2x - 8 = -2 2x = +6 x = +3
Therefore, the oxidation number of carbon in C₂O₄²⁻ is +3.
Understanding the +3 Oxidation State of Carbon
The +3 oxidation state for carbon is relatively uncommon compared to its more prevalent oxidation states of -4, +2, and +4. This unusual state arises due to the specific bonding within the oxalate ion. The structure of C₂O₄²⁻ involves two carbon atoms linked by a single bond, each carbon atom bound to two oxygen atoms via a double bond. Each carbon atom shares electrons with other atoms, resulting in a formal charge of +3 per carbon atom.
Comparing Oxidation States of Carbon
It’s beneficial to compare this oxidation state with others frequently exhibited by carbon:
-
-4: This is the most reduced state, commonly seen in methane (CH₄) and other alkanes. Carbon has four shared electrons, resulting in a neutral state.
-
+2: This oxidation state appears in carbon monoxide (CO) where carbon shares two electrons with the oxygen atom and has two non-bonding valence electrons.
-
+4: This is the most oxidized state, evident in carbon dioxide (CO₂), where the carbon atom shares four electrons with oxygen atoms, resulting in a complete octet.
The +3 oxidation state in C₂O₄²⁻ sits between +2 and +4, showcasing the versatility of carbon's bonding capabilities.
Oxidation Number vs. Formal Charge
It is crucial to distinguish between oxidation number and formal charge. While both involve assigning charges to atoms within a molecule, they differ conceptually.
-
Oxidation number is based on the assumption of completely ionic bonds, assigning electrons to the more electronegative atom in the bond. It's a useful bookkeeping tool for tracking electron transfer during redox reactions.
-
Formal charge considers the number of valence electrons an atom owns in a molecule. It is calculated by subtracting the number of non-bonding electrons and half the number of bonding electrons from the number of valence electrons in the free atom.
In C₂O₄²⁻, while the oxidation number of carbon is +3, the formal charge on each carbon atom will be different depending on the resonance structures considered. Resonance structures distribute the negative charges throughout the molecule, leading to a fractional formal charge. The oxidation number, however, provides a convenient way to track the overall electron distribution in the molecule.
Applications of Oxalate and its Significance
The oxalate ion (C₂O₄²⁻) has numerous applications across various fields, stemming from its ability to act as a bidentate ligand, meaning it can bind to a central metal ion through two donor oxygen atoms.
-
Coordination Chemistry: Oxalate is a vital ligand in coordination complexes, forming stable complexes with many transition metals. These complexes have varied applications in catalysis, material science, and medicine.
-
Biochemistry: Oxalate plays a crucial role in various biological processes. It's a component of certain metabolic pathways, and its metabolism can affect calcium levels in the body, potentially leading to kidney stones.
-
Analytical Chemistry: Oxalate is used in analytical techniques for the determination of several metal ions. Its ability to form precipitates with specific metal ions allows for quantitative analysis.
-
Industry: Oxalates have industrial applications, including in photography, textile processing, and metal treatment.
Common Misconceptions and Clarifications
Several common misconceptions surrounding the oxidation number of carbon in C₂O₄²⁻ warrant clarification:
-
Assuming equal oxidation states for both carbons: It's essential to remember that the oxidation number calculation considers the entire molecule. While both carbon atoms are structurally equivalent, the formal charges may differ slightly depending on the resonance structure considered. However, the oxidation number remains consistent across all resonance structures.
-
Confusing oxidation number with actual charge: Oxidation numbers are hypothetical charges and don't necessarily reflect the actual electron distribution in a molecule.
-
Ignoring the overall charge of the ion: The -2 charge of the oxalate ion is crucial in the calculation, ensuring that the sum of oxidation numbers of all atoms equals the total charge of the ion.
Conclusion
Determining the oxidation number of carbon in C₂O₄²⁻ involves applying the rules of oxidation number assignment systematically. The result, +3, highlights the versatile nature of carbon's bonding capacity. Understanding this oxidation state is crucial for comprehending the chemical behavior of oxalate and its significance in various chemical and biological systems. It is essential to distinguish between oxidation number and formal charge and avoid common misconceptions to correctly interpret the electronic structure and reactivity of this important ion. This understanding is fundamental for students and researchers in chemistry, biochemistry, and related fields. The applications of oxalates are extensive, reinforcing the importance of understanding their fundamental properties.
Latest Posts
Related Post
Thank you for visiting our website which covers about Oxidation Number Of C In C2o42- . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.