Oxidation No Of Cr In K2cr2o7
News Leon
Mar 20, 2025 · 5 min read
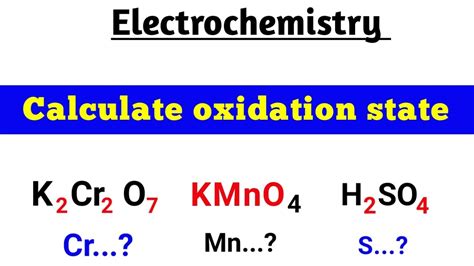
Table of Contents
Determining the Oxidation Number of Cr in K₂Cr₂O₇: A Comprehensive Guide
The determination of oxidation numbers is a fundamental concept in chemistry, crucial for balancing redox reactions and understanding the behavior of elements in compounds. This article delves into the process of calculating the oxidation number of chromium (Cr) in potassium dichromate (K₂Cr₂O₇), a powerful oxidizing agent frequently encountered in various chemical applications. We'll explore the step-by-step methodology, address common misconceptions, and discuss the significance of this oxidation state.
Understanding Oxidation Numbers
Before we tackle the specific case of K₂Cr₂O₇, let's briefly review the concept of oxidation numbers. The oxidation number, also known as the oxidation state, represents the hypothetical charge an atom would have if all bonds to atoms of different elements were completely ionic. It's a crucial tool for tracking electron transfer in chemical reactions.
Key Rules for Assigning Oxidation Numbers:
-
The oxidation number of an element in its free (uncombined) state is always zero. For example, the oxidation number of O₂ is 0, and the oxidation number of Na is 0.
-
The oxidation number of a monatomic ion is equal to its charge. For instance, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
-
The oxidation number of hydrogen is +1, except in metal hydrides where it is -1. Examples include HCl (+1 for H), and NaH (-1 for H).
-
The oxidation number of oxygen is -2, except in peroxides (like H₂O₂) where it is -1, and in superoxides (like KO₂) where it is -1/2. This is a crucial rule for our K₂Cr₂O₇ calculation.
-
The sum of the oxidation numbers of all atoms in a neutral molecule is zero.
-
The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
These rules provide a systematic approach for determining oxidation numbers in various compounds.
Calculating the Oxidation Number of Cr in K₂Cr₂O₇
Now, let's apply these rules to determine the oxidation number of chromium in potassium dichromate (K₂Cr₂O₇).
Step 1: Identify the known oxidation numbers.
- Potassium (K): Alkali metals always have an oxidation number of +1.
- Oxygen (O): In most compounds, oxygen has an oxidation number of -2. Since K₂Cr₂O₇ is not a peroxide or superoxide, we use -2 for oxygen.
Step 2: Assign variables.
Let's represent the oxidation number of chromium (Cr) as 'x'.
Step 3: Apply the rule of the sum of oxidation numbers.
Since K₂Cr₂O₇ is a neutral molecule, the sum of the oxidation numbers of all its atoms must be zero. We can express this mathematically:
2*(Oxidation number of K) + 2*(Oxidation number of Cr) + 7*(Oxidation number of O) = 0
Substituting the known oxidation numbers:
2(+1) + 2(x) + 7(-2) = 0
Step 4: Solve for x.
Simplifying the equation:
2 + 2x - 14 = 0
2x = 12
x = +6
Therefore, the oxidation number of chromium (Cr) in K₂Cr₂O₇ is +6.
Significance of the +6 Oxidation State of Chromium
The +6 oxidation state of chromium in K₂Cr₂O₇ is highly significant due to its strong oxidizing properties. This high oxidation state indicates that chromium is readily available to accept electrons, making it a powerful oxidizing agent. This characteristic is exploited in various applications, including:
-
Oxidative reactions in organic chemistry: K₂Cr₂O₇ is frequently used as an oxidizing agent in organic synthesis to convert alcohols to aldehydes or ketones, and aldehydes to carboxylic acids.
-
Analytical chemistry: Its strong oxidizing power makes it useful in titrations to determine the concentration of reducing agents.
-
Electroplating: Potassium dichromate can be employed in chromium electroplating processes, creating a durable and corrosion-resistant coating on metal surfaces.
-
Leather tanning: It plays a role in the tanning process, where it helps to convert animal hides into leather.
-
Photography: Potassium dichromate has historical uses in photographic processes due to its light sensitivity.
Common Misconceptions about Oxidation Numbers
It's essential to address some common misunderstandings related to oxidation numbers:
-
Oxidation numbers are not always equal to the actual charge: They are a hypothetical charge assigned based on certain rules and do not necessarily represent the real charge distribution in the molecule.
-
Oxidation numbers can be fractions: In certain compounds, such as superoxides, the oxidation numbers can be fractional values.
-
Oxidation numbers are not always integers: While many oxidation numbers are integers, they can be fractions in some cases, especially with complex molecules and unusual bonding.
-
The oxidation number is a bookkeeping tool: It's a convenient way to track the electron transfer during chemical reactions, facilitating the balancing of redox equations.
Deeper Dive: Redox Reactions and K₂Cr₂O₇
Potassium dichromate's role as an oxidizing agent is intrinsically linked to its chromium's +6 oxidation state. In redox reactions, K₂Cr₂O₇ undergoes reduction, meaning it gains electrons, with the chromium's oxidation state decreasing. This reduction is often accompanied by a color change, usually from orange to green, which makes it visually evident. The reduction of Cr⁶⁺ to Cr³⁺ is a commonly observed process.
Let’s consider a simple example of a redox reaction involving K₂Cr₂O₇: the oxidation of ethanol to ethanoic acid.
In this reaction, ethanol (CH₃CH₂OH) is oxidized to ethanoic acid (CH₃COOH), while potassium dichromate is reduced. The balanced equation demonstrates the electron transfer and the change in oxidation states:
3CH₃CH₂OH + 2K₂Cr₂O₇ + 8H₂SO₄ → 3CH₃COOH + 2Cr₂(SO₄)₃ + 2K₂SO₄ + 11H₂O
In this reaction, the chromium in K₂Cr₂O₇ goes from a +6 oxidation state to a +3 oxidation state in Cr₂(SO₄)₃. This change reflects the gain of electrons by the chromium atoms, confirming K₂Cr₂O₇’s role as an oxidizing agent.
Conclusion
The determination of the oxidation number of chromium in K₂Cr₂O₇, which is +6, provides critical insights into the compound's chemical behavior and its extensive use as a powerful oxidizing agent. Understanding oxidation numbers is crucial for mastering redox chemistry, balancing equations, and appreciating the reactivity of various chemical species. By applying the fundamental rules for assigning oxidation numbers and comprehending the significance of the +6 oxidation state of chromium, we can better understand the chemistry of potassium dichromate and its diverse applications. Remember that consistently practicing these rules and understanding their rationale will strengthen your grasp of this core concept in chemistry.
Latest Posts
Latest Posts
-
Where Is The International Court Of Justice Situated
Mar 21, 2025
-
The Sum Of Three Consecutive Numbers Is 72
Mar 21, 2025
-
As A Runaway Scientific Balloon Ascends
Mar 21, 2025
-
1000 Days In Years And Months
Mar 21, 2025
-
A Thin Circular Disk Of Radius R
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Oxidation No Of Cr In K2cr2o7 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
