Orbitals With The Same Energy Are Called
News Leon
Mar 26, 2025 · 6 min read
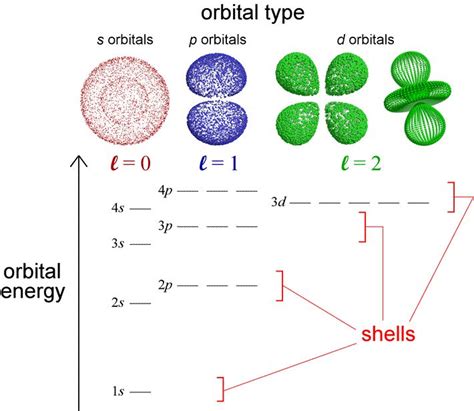
Table of Contents
Orbitals with the Same Energy are Called Degenerate Orbitals: A Deep Dive into Atomic Structure
Orbitals are regions of space around an atom's nucleus where there's a high probability of finding an electron. Understanding how these orbitals are arranged and their energy levels is crucial to grasping the fundamentals of chemistry. A key concept in this understanding is degeneracy, which describes orbitals possessing the same energy level. This article delves deep into the concept of degenerate orbitals, exploring their characteristics, the factors influencing degeneracy, and the implications for atomic and molecular behavior.
What are Degenerate Orbitals?
Orbitals with the same energy are called degenerate orbitals. This means that electrons occupying these orbitals possess identical energy levels within a given electron shell or subshell. The term "degenerate" comes from the Latin "degenerare," meaning "to fall from a higher to a lower state," reflecting the potential for these orbitals to split in energy under certain conditions. Imagine them as equally comfortable places for an electron to reside within the atom.
Key Characteristics of Degenerate Orbitals:
- Same Principal Quantum Number (n): Degenerate orbitals always share the same principal quantum number, n. This number determines the energy level and size of the orbital.
- Same Subshell (l): They also belong to the same subshell, defined by the azimuthal quantum number, l. The subshell determines the shape of the orbital (s, p, d, f).
- Different Magnetic Quantum Number (ml): While sharing the same n and l, degenerate orbitals differ in their magnetic quantum number, ml. This quantum number specifies the orientation of the orbital in space. For instance, the three p orbitals (px, py, pz) within a p subshell are degenerate because they have the same energy, but they are oriented along different axes in three-dimensional space.
Factors Affecting Orbital Degeneracy
The degeneracy of orbitals isn't a fixed, immutable property; it's sensitive to several factors. Let's explore these influences:
1. The Hydrogen Atom: A Perfect Example of Degeneracy
The hydrogen atom provides the simplest model to understand orbital degeneracy. In a hydrogen atom, orbitals with the same principal quantum number (n) but different azimuthal quantum numbers (l) are degenerate. For example, the 2s and 2p orbitals have the same energy, regardless of their different shapes. This perfect degeneracy arises from the simplicity of the hydrogen atom's single proton and electron interaction – there is no electron-electron repulsion to lift the degeneracy.
2. Multi-Electron Atoms: Lifting the Degeneracy
The situation changes drastically in multi-electron atoms. The presence of multiple electrons introduces electron-electron repulsion. This repulsion significantly alters the energy levels of the orbitals. The electrons are not only influenced by the attractive force of the nucleus, but they also repel each other, resulting in a more complex energy landscape.
This electron-electron repulsion leads to the lifting of degeneracy. Orbitals that were previously degenerate become non-degenerate, meaning their energies are no longer identical. The extent of this degeneracy lifting depends on the effective nuclear charge experienced by each electron and the spatial overlap between electron orbitals.
Illustrative Example: 2p Orbitals in a Multi-Electron Atom
In a multi-electron atom, the three 2p orbitals (2px, 2py, 2pz) which are degenerate in a hydrogen atom, are no longer exactly degenerate. Their energies are slightly different due to the variations in electron-electron repulsion experienced by electrons in these orbitals.
3. External Electric and Magnetic Fields: Stark and Zeeman Effects
Applying external electric or magnetic fields can also lift the degeneracy of atomic orbitals.
- Stark Effect: This is the splitting of spectral lines in the presence of an external electric field. The electric field perturbs the electron's potential energy, causing the energy levels of the orbitals to shift slightly.
- Zeeman Effect: This phenomenon is the splitting of spectral lines in the presence of an external magnetic field. The magnetic field interacts with the electron's spin and orbital angular momentum, modifying its energy levels and leading to the lifting of degeneracy.
These effects are particularly significant in spectroscopic studies, where they provide valuable insights into the structure and properties of atoms and molecules.
Implications of Degenerate Orbitals
The concept of degenerate orbitals has profound implications in various aspects of chemistry:
1. Electronic Configuration and Hund's Rule
When filling degenerate orbitals with electrons, Hund's rule comes into play. This rule states that electrons will individually occupy each degenerate orbital with parallel spins before pairing up in the same orbital. This maximizes the total spin of the atom and minimizes electron-electron repulsion. For instance, in the nitrogen atom's p subshell, each of the three 2p orbitals is singly occupied before any pairing begins.
2. Molecular Orbital Theory
Degeneracy plays a critical role in molecular orbital theory. When atoms combine to form molecules, their atomic orbitals interact to form molecular orbitals. The energy levels of these molecular orbitals are influenced by the degeneracy of the atomic orbitals from which they originate. Degenerate atomic orbitals often combine to form degenerate molecular orbitals, leading to bonding and anti-bonding orbitals with the same energy.
3. Spectroscopy
The degeneracy of atomic orbitals is directly observable through spectroscopic techniques. The absorption or emission of electromagnetic radiation can result in transitions between different energy levels, with the splitting of spectral lines revealing information about the degeneracy and the lifting of it due to the presence of external fields or electron-electron interaction.
4. Catalysis
The concept of degenerate orbitals, and their splitting under external influence, plays a key role in the field of catalysis. Catalytic processes often involve the interaction between a substrate molecule and a catalyst surface, which creates perturbed energy levels for the orbitals of the substrate, allowing for reactions to occur more efficiently than under non-catalytic conditions.
Beyond Atomic Orbitals: Degeneracy in Other Systems
The idea of degeneracy isn't limited to atomic orbitals. It's a broader concept applicable to various quantum mechanical systems. For example:
- Vibrational modes in molecules: Molecules exhibit different vibrational modes, and some of these modes might have the same energy, resulting in degenerate vibrational levels.
- Nuclear energy levels: Nuclear energy levels can also exhibit degeneracy, significantly affecting nuclear reactions and radioactive decay processes.
Conclusion: The Significance of Degenerate Orbitals
The concept of degenerate orbitals, while seemingly abstract, is fundamental to understanding the structure and behavior of atoms, molecules, and other quantum systems. The presence and lifting of degeneracy significantly impact electronic configurations, molecular properties, and various spectroscopic phenomena. It underscores the complex interplay of attractive and repulsive forces within these systems and highlights the importance of electron-electron interactions in shaping the energy landscapes of multi-electron atoms. As we continue to explore the intricacies of quantum mechanics, the understanding of orbital degeneracy remains crucial for further advancements in chemistry, physics, and materials science. The ability to predict and manipulate orbital degeneracy opens exciting possibilities for designing new materials and controlling chemical reactions with increased precision.
Latest Posts
Latest Posts
-
A Garden Hose With An Internal Diameter
Mar 29, 2025
-
Earth Is Approximately A Sphere Of Radius 6 37
Mar 29, 2025
-
Is Salt Water A Heterogeneous Mixture
Mar 29, 2025
-
What Is The Opposite Word Of Innocent
Mar 29, 2025
-
How Much Days Is 48 Hours
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Orbitals With The Same Energy Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
